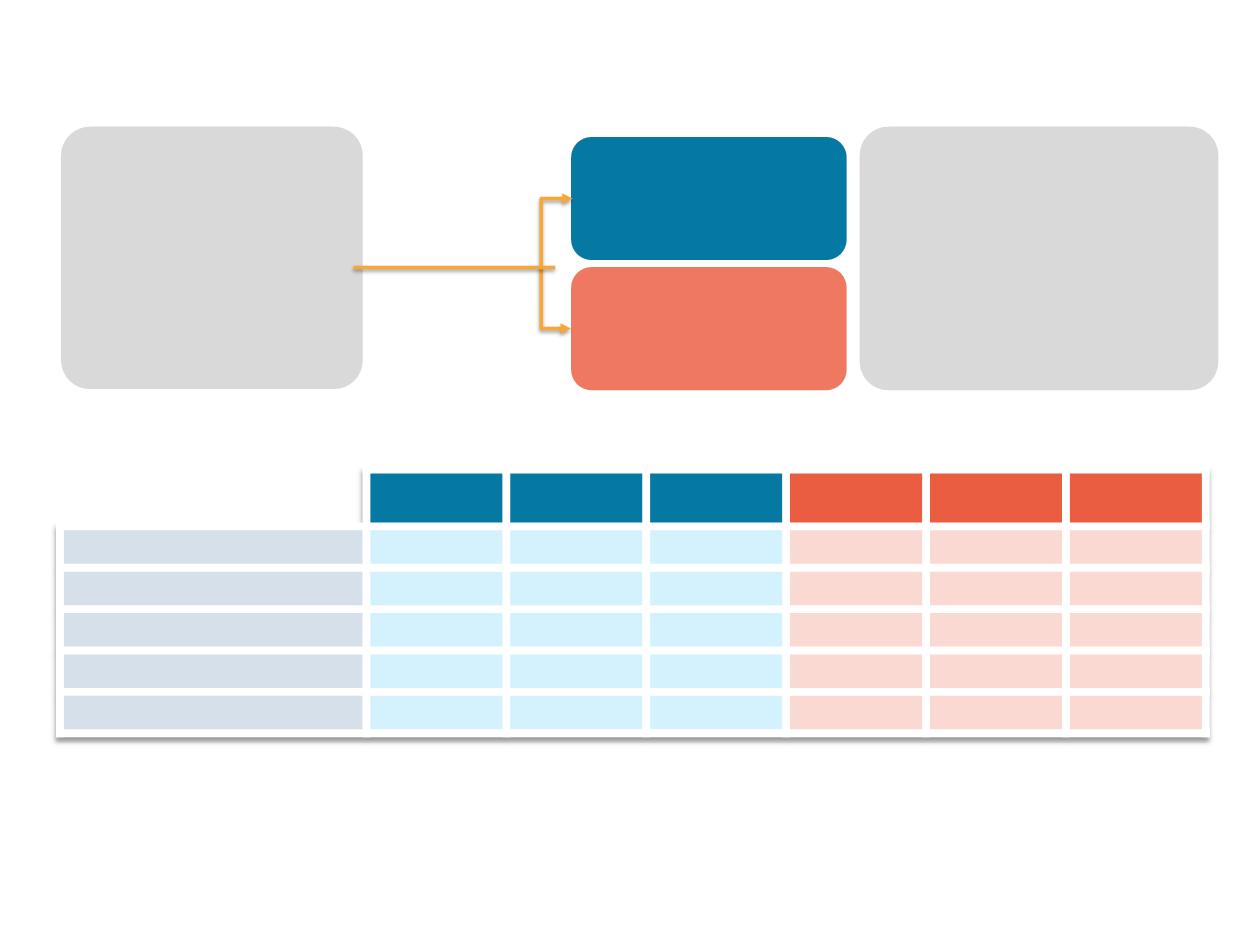
Phase III MONALEESA-2
Hortobagyi G, et al. NEJM 2016
Randomization (1:1)
Stratified by the
presence/absence
of liver and/or lung
metastases
Ribociclib (600 mg/day)
3-weeks-on/1-week-off
+
Letrozole (2.5 mg/day)
n=334
Placebo
+
Letrozole (2.5 mg/day)
n=334
Primary endpoint
•
PFS (locally assessed per
RECIST v1.1)
Secondary endpoints
•
Overall survival (key)
•
Overall response rate
•
Clinical benefit rate
•
Safety
•
Postmenopausal women
with HR+/HER2–
advanced breast cancer
•
No prior therapy for
advanced disease
•
N=668
Febrile neutropenia occurred in 5 (1.5%)* patients in the ribociclib arm vs.
none in the placebo arm
Adverse Event
≥5% in Either Arm, %
Ribociclib + Letrozole
n=334
Placebo + Letrozole
n=330
All
Grade 3
Grade 4
All
Grade 3
Grade 4
Neutropenia
74
50
9.6
5.2
0.9
0
Leukopenia
33
20
1.2
3.9
0.6
0
Anemia
19
0.9
0.3
4.5
1.2
0
Lymphopenia
11
5.7
1.2
2.1
0.9
0
Thrombocytopenia
9.0
0.6
0
0.6
0
0


