40
P. TERRA: Efficacy results
Kim T, et al. ESMO 2016. Abstract 465PD (poster discussion session).
Response, n (%)
Trifluridine/tipiracil
(n=261)
Placebo
(n=130)
CR
0
0
PR
3 (1.1)
0
SD
112 (42.9)
19 (14.6)
PD
125 (47.9)
95 (73.1)
Not evaluable
21 (8.0)
16 (12.3)
ORR
3 (1.1)
0
DCR
115 (44.1)
19 (14.6)
CI = confidence interval; CR = complete response; DCR = disease control rate (CR + PR + SD); HR =
hazard ratio; ORR = overall response rate (CR + PR); PD = progressive disease; PR = partial
response; SD = stable disease
OS
PFS
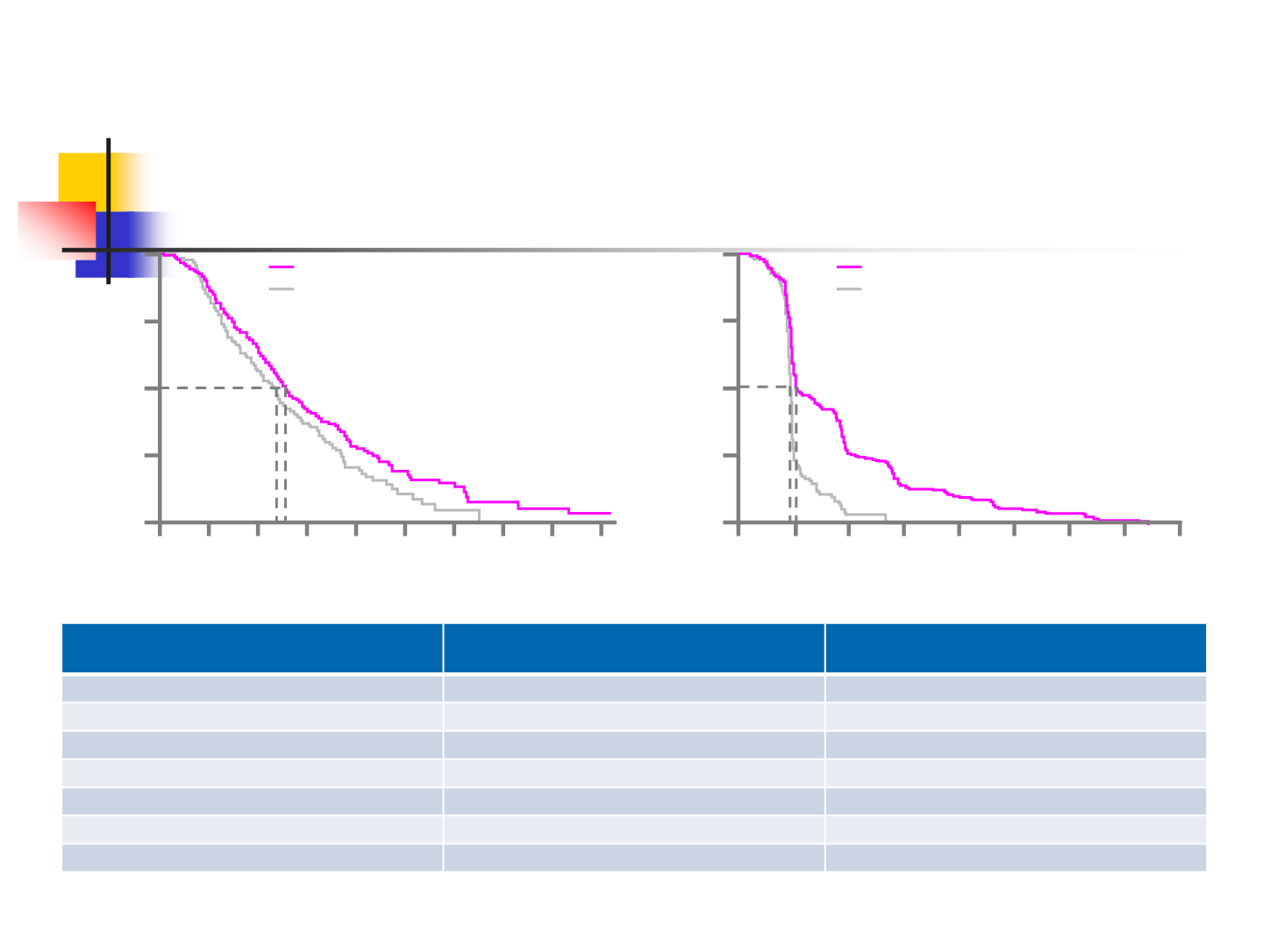
LONSURF showed a statistically significant increase in OS and PFS
0 3 6 9 12 15 18 21 24 27
Time (months)
0
25
50
75
100
% survival
7.1
7.8
1.8
2.0
LONSURF (n=271; 205 events)
Placebo (n=135; 111 events)
HR=0.79 (95% CI: 0.62, 0.99),
p=0.035
LONSURF (n=271; 249 events)
Placebo (n=135; 123 events)
HR=0.43 (95% CI: 0.34, 0.54),
p<0.001
75
100
0
2
4
6
8 10 12
16
Months from Randomization
0
25
50
% event free
14


