41
TERRA: Safety results and conclusion
BSC = best supportive care; TEAE = treatment-emergent adverse event
Kim T, et al. ESMO 2016. Abstract 465PD (poster discussion session).
LONSURF
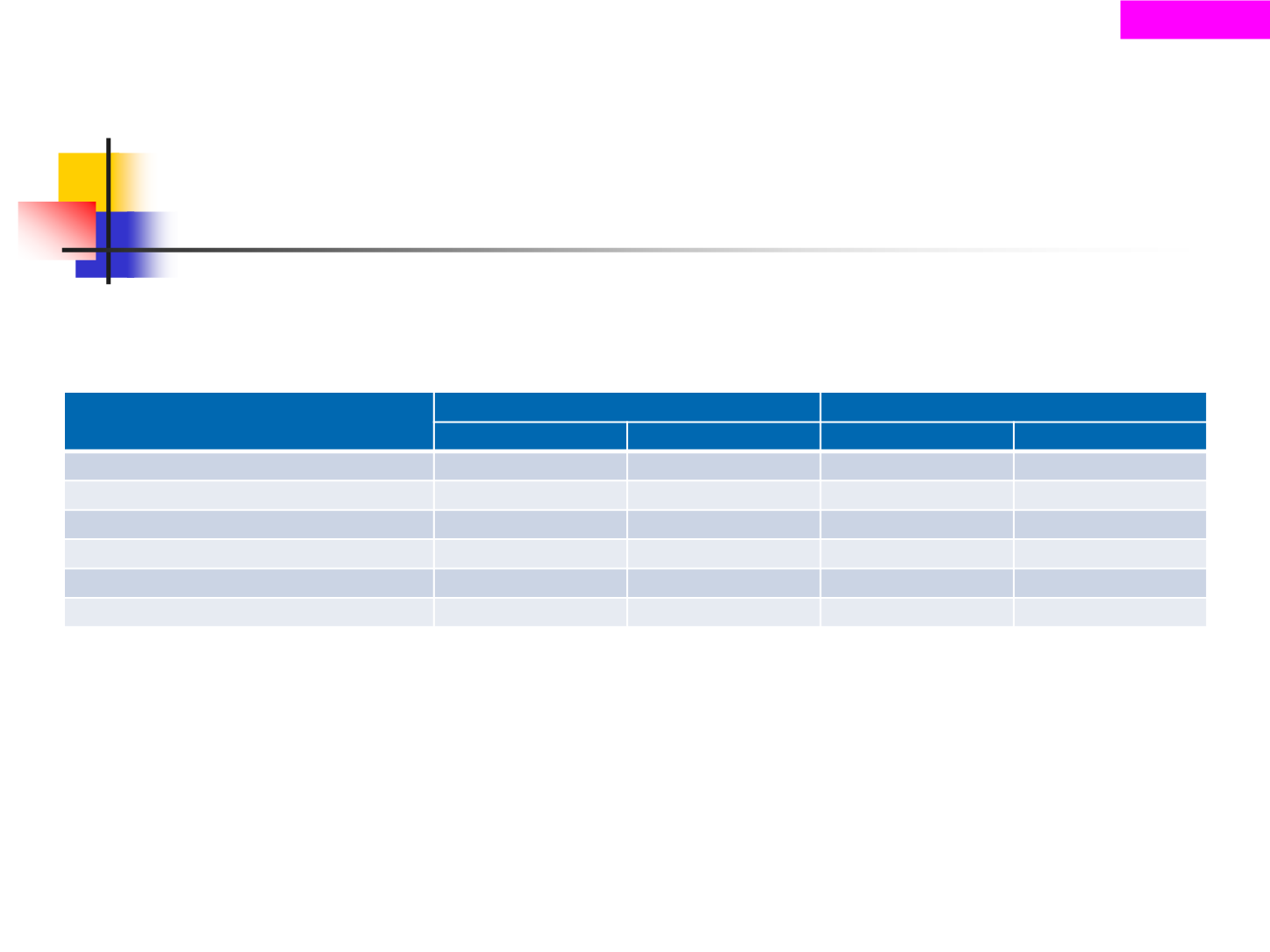
Selected TEAEs >20% for all
grades, n (%)
LONSURF + BSC (n=271)
Placebo + BSC (n=135)
All grades
Grade ≥3
All grades
Grade ≥3
Anemia
124 (45.8)
43 (15.9)
27 (20.0)
8 (5.9)
Nausea
104 (38.4)
2 (0.7)
20 (14.8)
1 (0.7)
Neutropenia
98 (36.2)
55 (20.3)
1 (0.7)
0
Decreased appetite
80 (29.5)
2 (0.7)
22 (16.3)
3 (2.2)
Fatigue
69 (25.5)
6 (2.2)
19 (14.1)
4 (3.0)
Vomiting
60 (22.1)
3 (1.1)
13 (9.6)
1 (0.7)
The safety profile of LONSURF was as expected with haematological toxicities more common compared with
placebo + BSC


