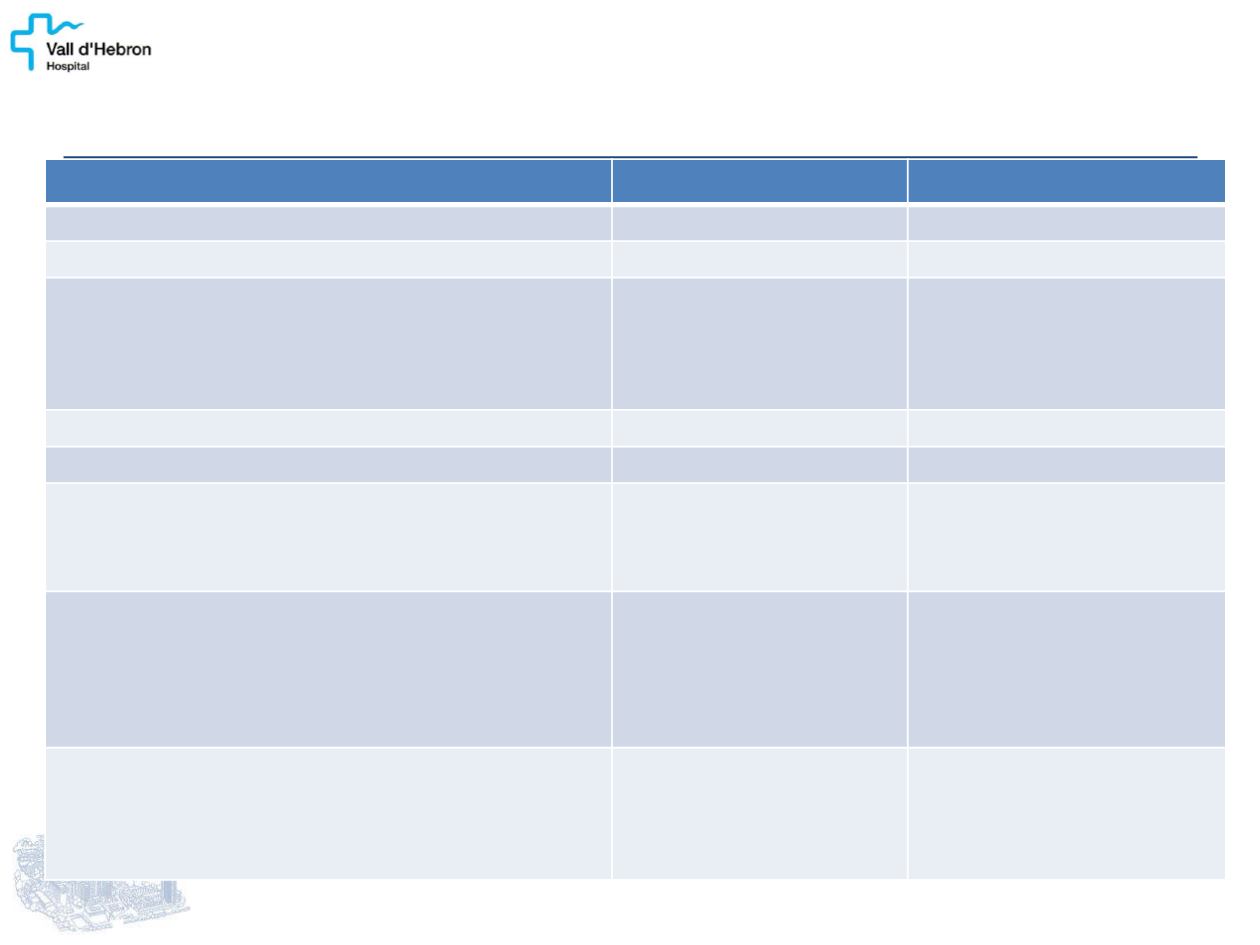
CLARINET: BASELINE CHARACTERISTICS
Lanreotide (n=101)
Placebo (n=103)
Men
, n (%)
53 (52)
54 (52)
Age
in years, mean (SD)
63.3 (9.8)
62.2 (11.1)
NET origin
, n (%)
Pancreas
Midgut
Hindgut
Unknown/other
42 (42)
33 (33)
11 (11)
15 (15)
49 (48)
40 (39)
3 (3)
11 (11)
Tumour progression
, n (%)
4 (4)
5 (5)
Prior treatment
, n (%)
16 (16)
16 (16)
Tumour grade
, n (%)*
1 (Ki-67: 0–2%)
2 (Ki-67: 3–10%)
Unknown
69 (68)
32 (32)
0
72 (70)
29 (28)
2 (2)
Hepatic tumour volume
, n (%)
0%
>0–10%
>10–25%
>25–50%
>50%
16 (16)
33 (33)
13 (13)
23 (23)
16 (16)
18 (17)
40 (39)
17 (17)
12 (12)
16 (16)
Chromogranin A,
n (%)
≤1 × ULN
1–2 × ULN
>2 × ULN
Unknown
33 (33)
25 (25)
41 (41)
2 (2)
34 (33)
18 (17)
48 (47)
3 (3)
*Ki-67 thresholds as per World Health Organization (WHO) 2010 classification with values >2–≤10% assigned to grade 2.
Caplin ME, et al.
N Engl J Med.
2014


