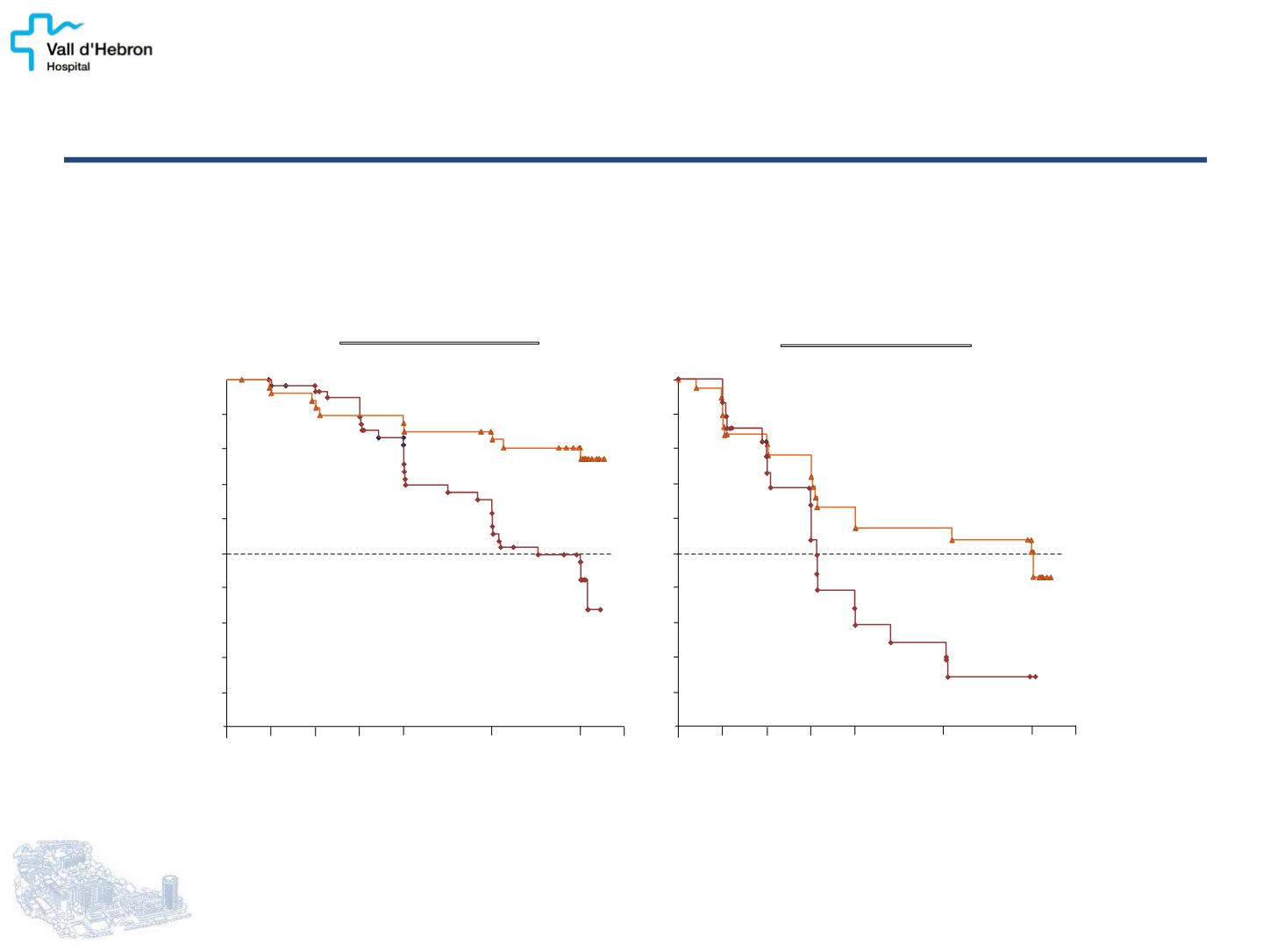
PFS low vs high hepatic tumor load
P
value derived from log-rank test; HR derived from Cox proportional hazards model; NC, not calculable
Lanreotide Autogel
vs p lacebo
P
=.0002
HR=0.34 [95% CI: 0.18, 0.62]
Tumor
load >25%
(n =67)
Lanreotide Autogel
vs
placebo
P
=
.0170
HR=0.45 [95% CI: 0.23, 0.88]
0
10
20
30
40
50
60
70
80
90
100
Time, months
Lanreotide Autogel
120 mg
14 events
/
62 patients
median, not
reached
Placebo
41 events / 75 patients
median, 21.1 months [95% CI: 17.6, 24.4]
0 3 6 9 12
18
24 27
0
10
20
30
40
50
60
70
80
90
100
Time, months
Lanreotide
A
utogel
120 mg
18 events
/ 39 patients
median, 24.1 months
[95% CI:
9.3, NC]
Placebo
19 events / 28 patients
median, 9.4 months [95% CI: 6.3, 12.0]
0 3 6 9 12
18
24 27
Patients Alive and With No Progression, %
Tumor load 25% (n=137)
Caplin ME, et al.
N Engl J Med.
2014
CLARINET: LANREOTIDE PROLONG PFS IN
ENTEROPANCREATIC NET


