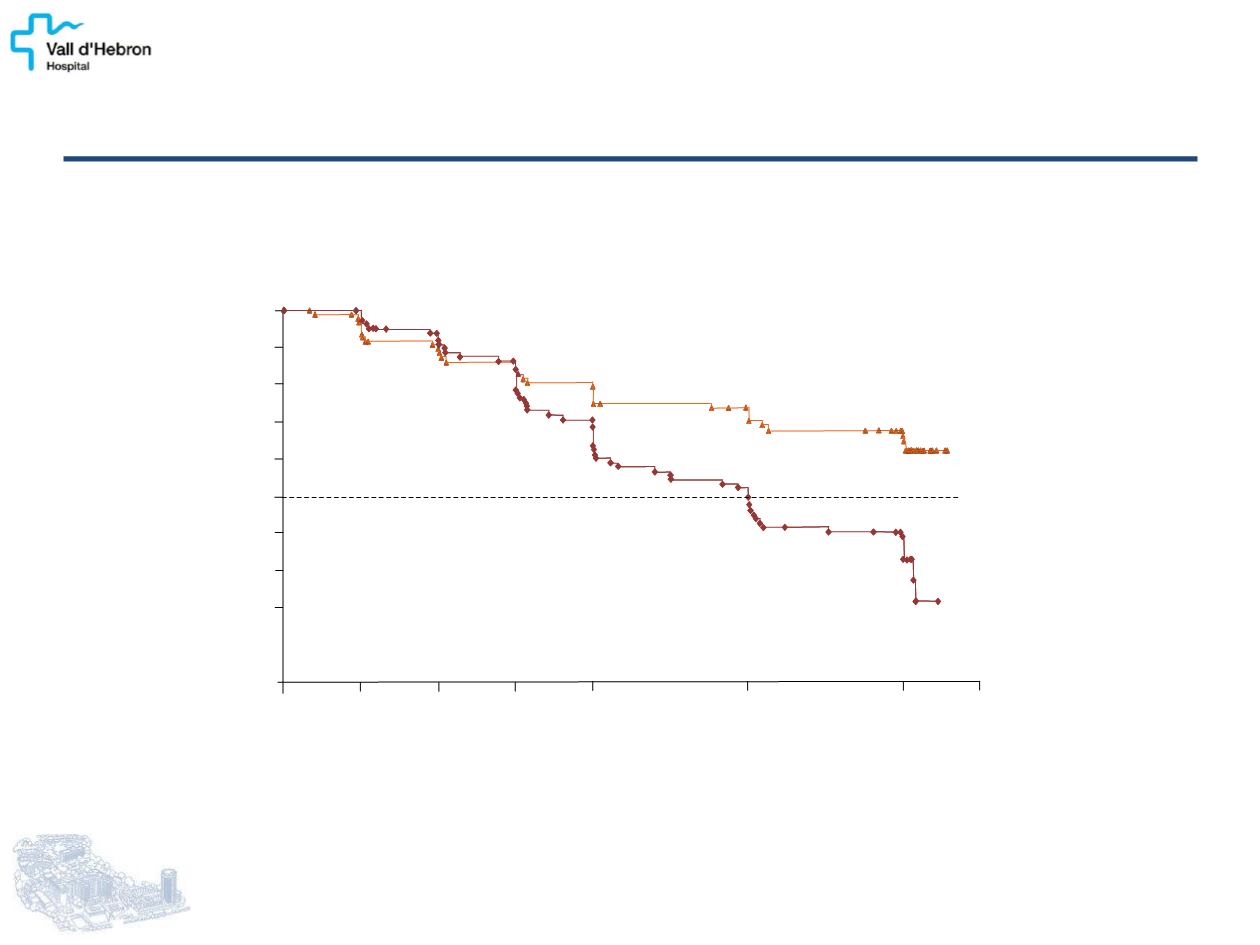
PFS
(intention to treat population)
101
94
84
78
71
61
103
101
87
76
59
43
40
No at risk:
26
0
0
CLARINET: LANREOTIDE PROLONG PFS IN
ENTEROPANCREATIC NET
0
3
6
9
12
18
24
27
0
10
20
30
40
50
60
70
80
90
100
62%
22%
Lanreotide Autogel 120 mg
32 events / 101 patients
median, not reached
Placebo
60 events / 103 patients
median, 18.0 months [95% CI: 12.1, 24.0]
Time, months
Patients Alive and With No Progression, %
Lanreotide Autogel vs Placebo
P = .0002 HR = 0.47 [95% CI: 0.30, 0.73]
Caplin ME, et al.
N Engl J Med.
2014


