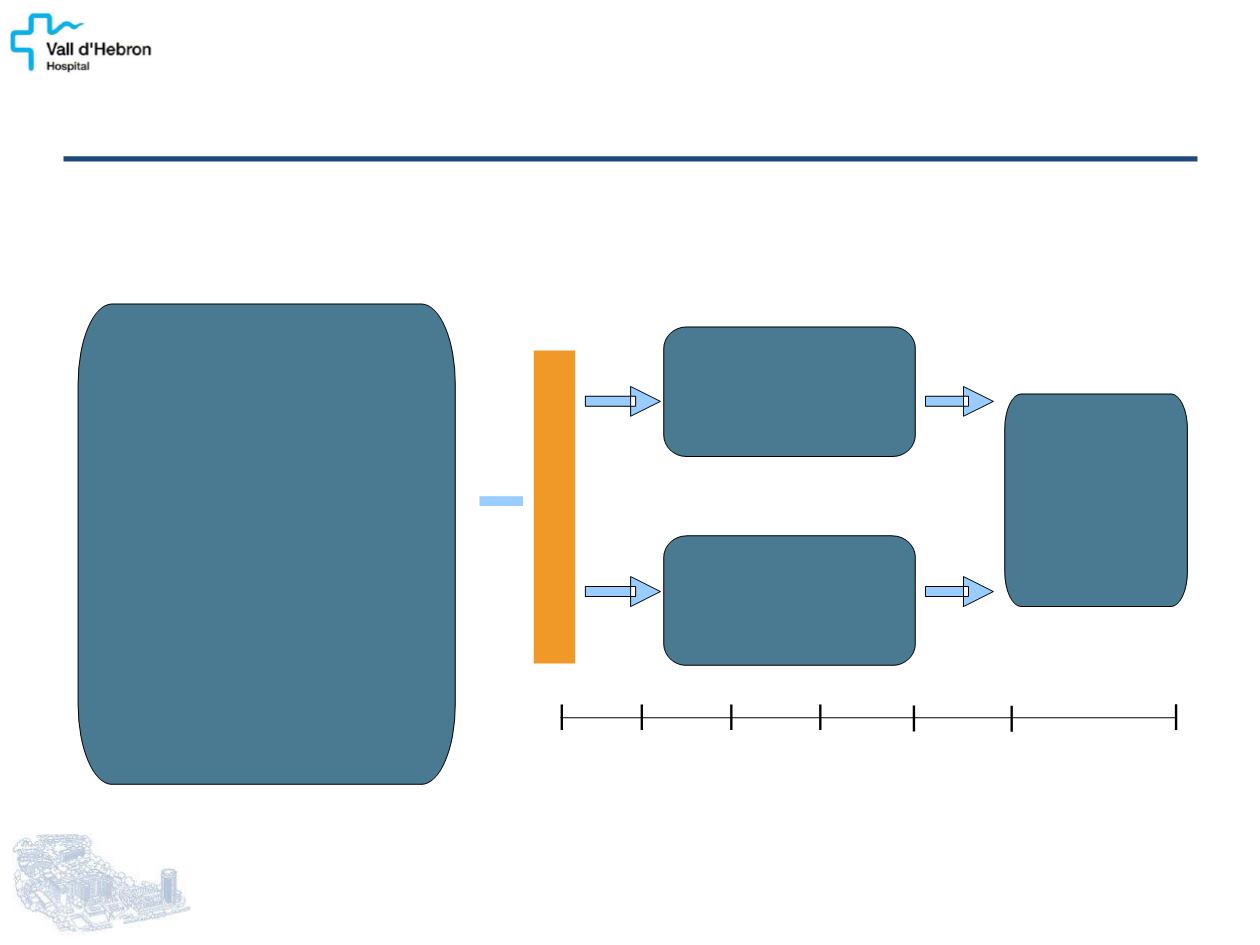
A randomized double-blind placebo-
C
ontrolled phase III study of
L
anreotide
A
ntiproliferative
R
esponse
I
n enteropancreatic
NET
Caplin ME, et al.
N Engl J Med.
2014
weeks
12
24
36
48
72
96
n = 204
RANDOMIZATION
(1:1)
ClinicalTrials.gov NCT00353496
EudraCT 2005-004904 -35
Patients with GEP-NET
•
Histologically confirmed
•
Measurable (CT / MRI)
•
Grade 1 / grade 2
well / mod differentiated
(Ki67 <10%) [WHO 2010
classification]
•
Locally inoperable or
metastatic
•
Nonfunctioning only
Lanreotide Autogel
120 mg sc
every 28 days
Placebo
every 28 days
Treatment
continued
until tumor
progression
or death
•
Primary endpoint: PFS
•
Secondary endpoints: Adverse events,
pharmacokinetics, quality of life, CgA serum levels
THE CLARINET STUDY


