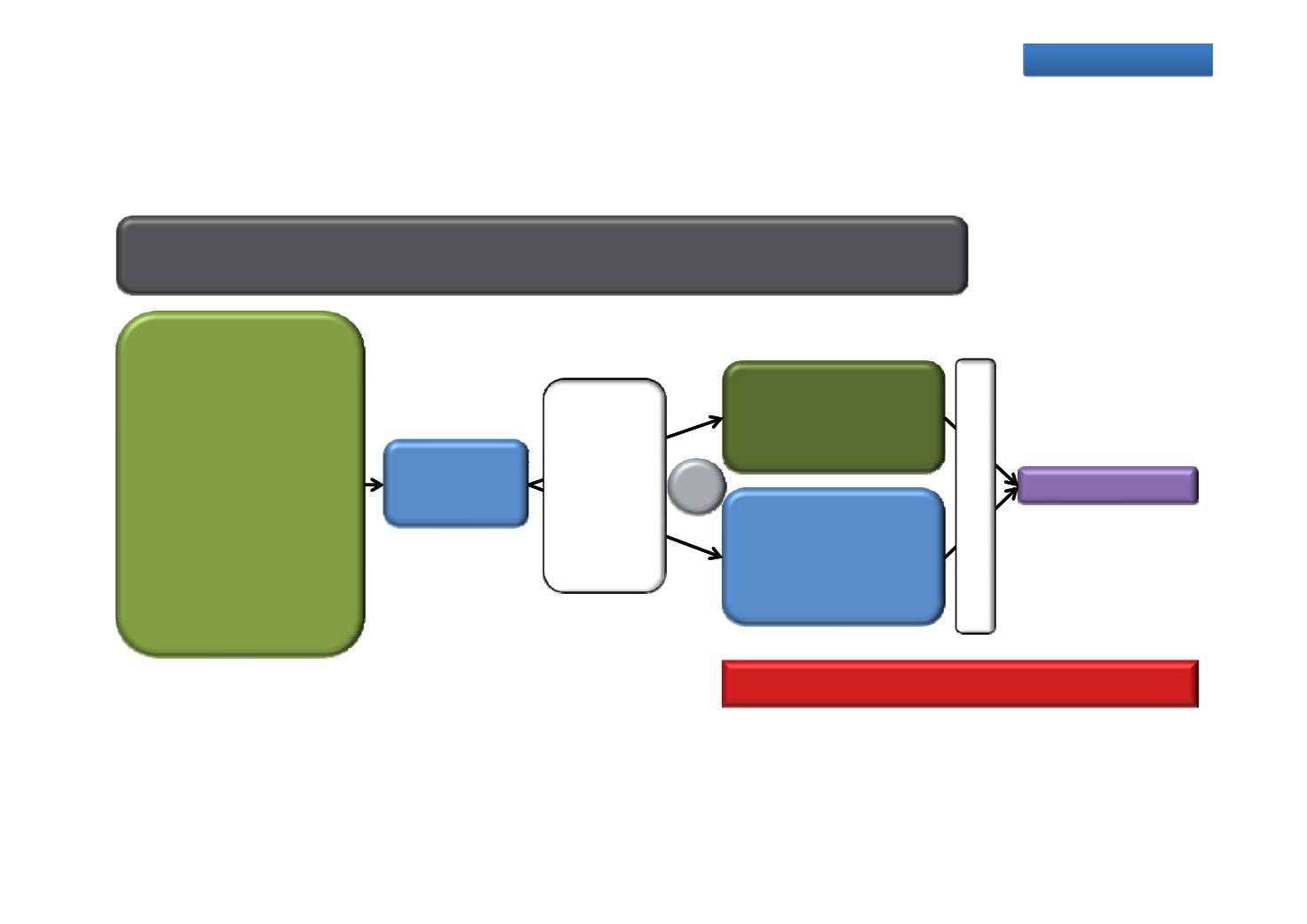
PRESIDE: Continued enzalutamide treatment
beyond progression in combination with docetaxel
Ongoing trials
in chemotherapy-naïve mCRPC
1
•
A Phase 3b randomised, double‐blind, placebo‐controlled study
•
Primary endpoint: PFS*
n=650
Chemotherapy‐naïve
mCRPC after ADT
failure
Testosterone
Open label
Placebo QD +
docetaxel 75 mg/m
2
/
3 weeks + prednisone (5
mg BID)
PSA
AND/OR
PR
O
GR
≤50 ng/dL
Progressive disease
with ongoing ADT
As mptomatic/
‐
enzalutamide
160 mg QD
Safety follow‐up
Enzalutamide
(160 mg QD) +
docetaxel 75 mg/m
2
/
RADIO‐
GRAPHIC
PROGRE‐
SSION
R
1:1
ES
S
I
O
y
mildly symptomatic
ECOG PS 0–1
2
3 weeks + prednisone (5
mg BID)
N
Planned evaluations
•
PFS*, time to PSA progression, PSA response, objective response rate,
time to pain progression, time to opiate use, time to first SRE, QoL
Recruiting
*Radiographic, unequivocal clinical progression, or death.
ADT=androgen‐deprivation therapy; BID=twice daily; ECOG PS=Eastern Cooperative Oncology Group performance status; mCRPC=metastatic castration‐
resistant prostate cancer; PFS=progression‐free survival; PSA=prostate‐specific antigen; QD=once daily; QoL=quality of life; R=randomisation; SRE=skeletal‐
related event.
1. Chowdhury S,
et al.
ASCO 2015; Poster presentation 70a; 2. NCT02288247. Available at
/. Last accessed: May 2015
2
3


