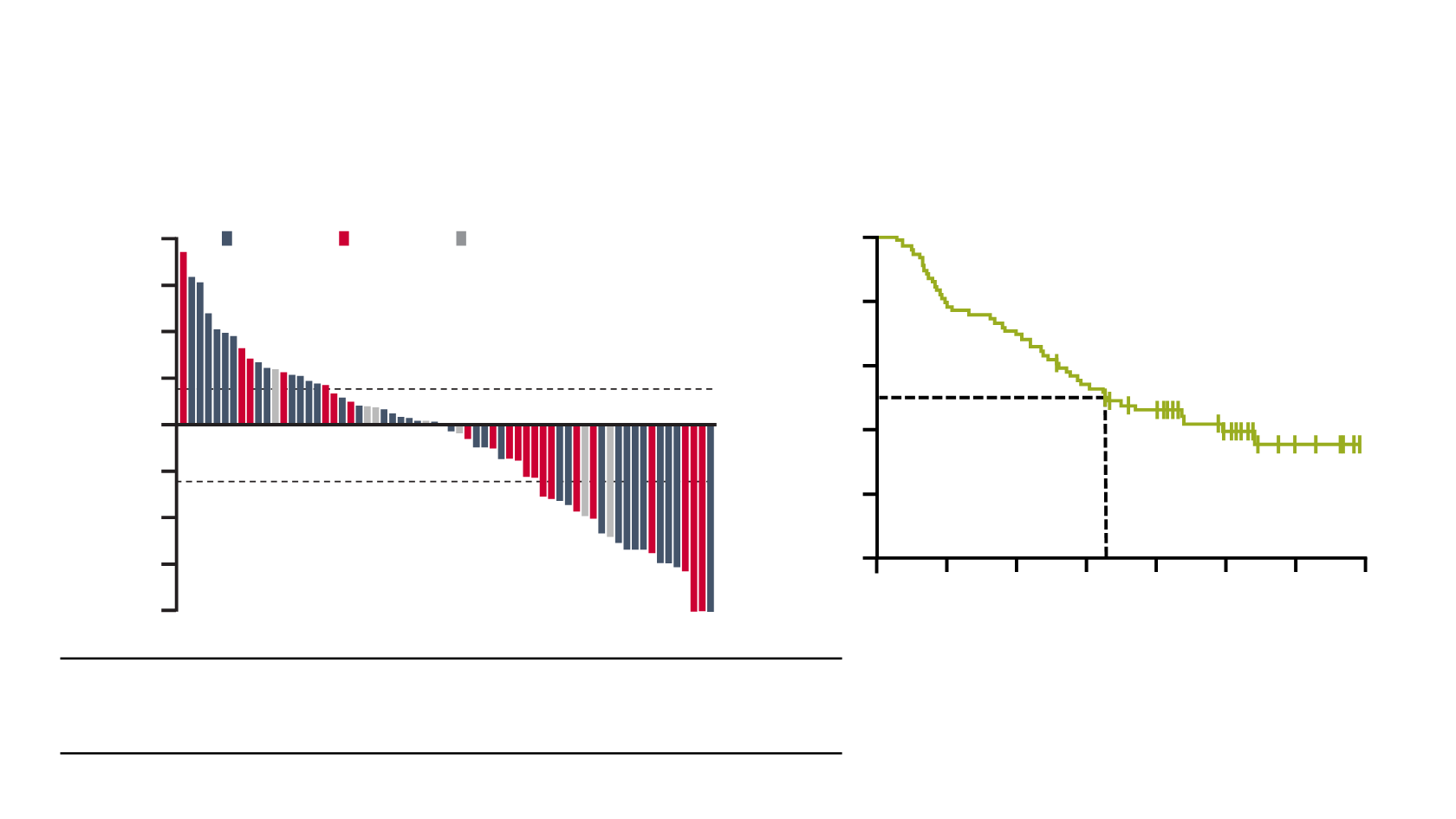
Nivolumab (CheckMate 032): tumour burden
reduction and OS
Nivolumab (N=78)
Confirmed ORR, % (95% CI)
24.4 (15.3–35.4)
CR, %
6.4
Confirmed ORR, % (95% CI) PD-L1 <1% / PD-L1 ≥1%
26.2 (13.9–42.0) / 24.0 (9.4–45.1)
*Complete/partial responses
Evaluable patients with target lesion at baseline and at least on-treatment tumour assessment
Sharma et al. ASCO 2016
* * * * *
* * *
* * * *
* * * *
* * *
PD-L1 ≥1%
PD-L1 not quantifiable
PD-L1 <1%
100
75
50
25
0
–25
–50
–75
–100
Best reduction from baseline
in target lesion (%)
Tumour burden reduction in target lesions
OS
OS estimate
Time (months)
9.72
(7.26–16.16)
12-month OS rate = 45.6%
0.2
0.8
0.0
0.4
0.6
1.0
0
3
6
9
12
15
18
21


