Current Treatment options approved in EU
BCG, Bacillus Calmette-Guérin; ORR, objective response rate; mOS, median overall survival; TURBT, transurethral resection of bladder tumor.
1. Babjuk M et al. Non-muscle-invasive bladder cancer guidelines.
EAU
. 2016. 2. Witjes JA et al. Muscle-invasive and Metastatic Bladder Cancer.
EAU
. 2016. 3. Bellmunt J et al.
Ann Oncol
. 2014;25(suppl 3):iii40-iii48. 4.
Sternberg CN et al.
Cancer
. 1989;64(12):2448-2458. 5. von der Maase H et al.
J Clin Oncol.
2000;18(17):3068-3077. 6. Roberts JT et al.
Ann Oncol
. 2006;17(suppl 5):v118-v122. 7. Sternberg CN et al.
Eur J Cancer
.
2006;42(1):50-54. 8. Dreicer R et al.
Cancer
. 2004;100(8):21639-1645. 9. Kaufman D et al.
J Clin Oncol.
2000;18(9):1921-1927. 10. Bellmunt J et al.
J Clin Oncol
. 2012;30(10):1107-1113. 11. De Santis M et al.
J Clin
Oncol
. 2012;30(2):191-199. 12. Dreicer R et al.
Cancer
. 2004;100(8):1639-1645. 13. Galsky MD et al.
Cancer
. 2007;109(3):549-555. 14. Bellmunt J et al.
J Clin Oncol
. 2009;27(27):4454-4461. 15. McCaffrey JA et al.
J
Clin Oncol
. 1997;15(5):1853-1857. 16. Vaughn DJ et al.
J Clin Oncol
. 2002;20(4):937-940.
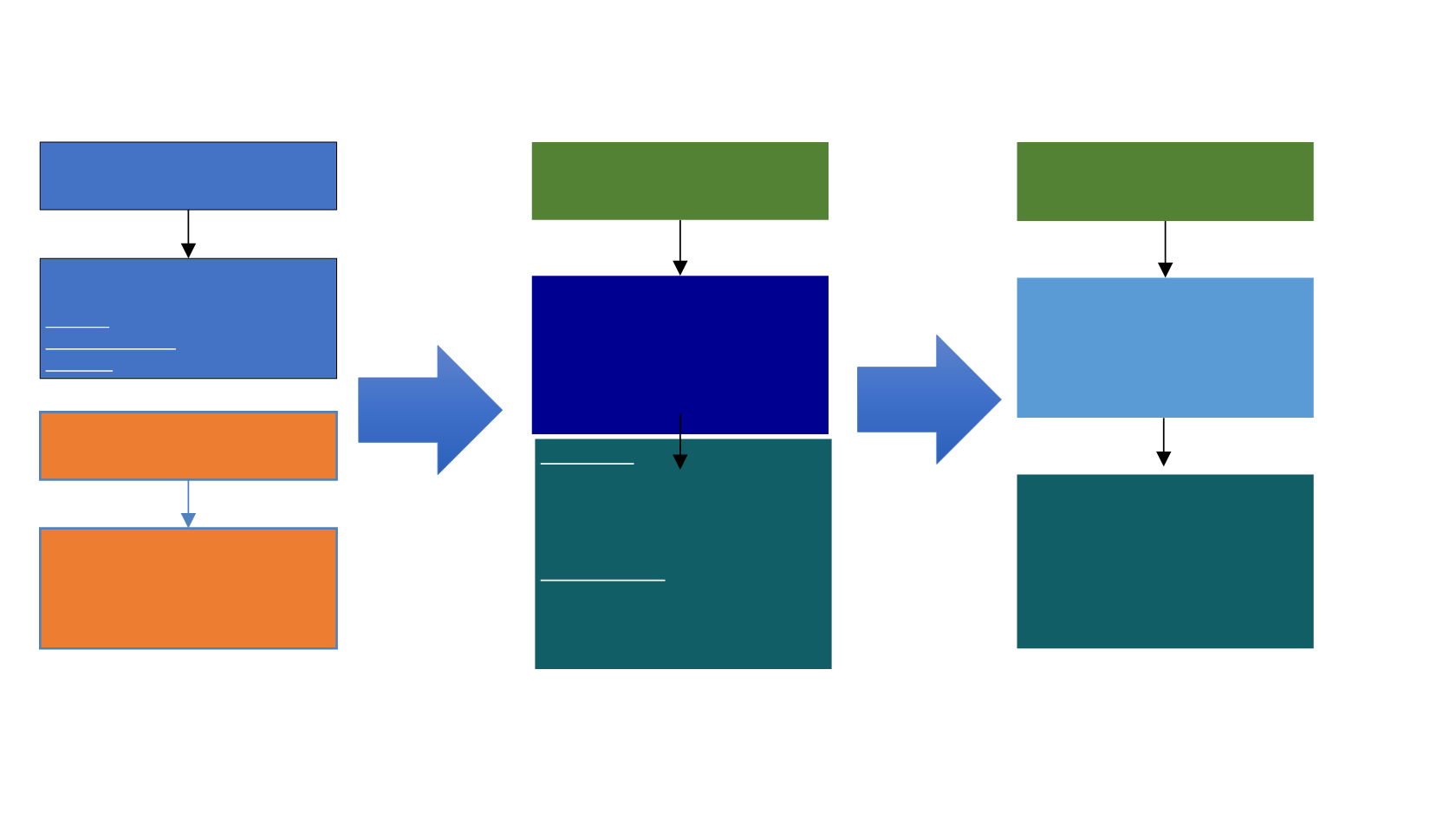
Non-Muscle Invasive
1
TURBT
Intravesical Therapy
Low Risk: Chemotherapy
Intermediate Risk: BCG
High Risk: Cystectomy
Muscle Invasive
2,3
Radical cystectomy + Neoadjuvant
cisplatin based regimens
≥ 2
nd
Line Metastatic
•
Vinflunine
•
Taxane-based CT
•
Clinical trial
ORR 8.6 - 13.3%
mOS 6.9 - 9.0 mo
1-year OS:~26%
1
st
Line
Metastatic
•
Cisplatin-based
combination (eg, MVAC,
Gem-Cis ± Pax)
•
Carboplatin-based
regimens
Cisplatin:
ORR:~28-72%
mOS:~13-16 mo
1 year OS :~54-63%
Carboplatin:
ORR:~28-56%
mOS:~8-15 mo
1-year OS:~33-54%
6


