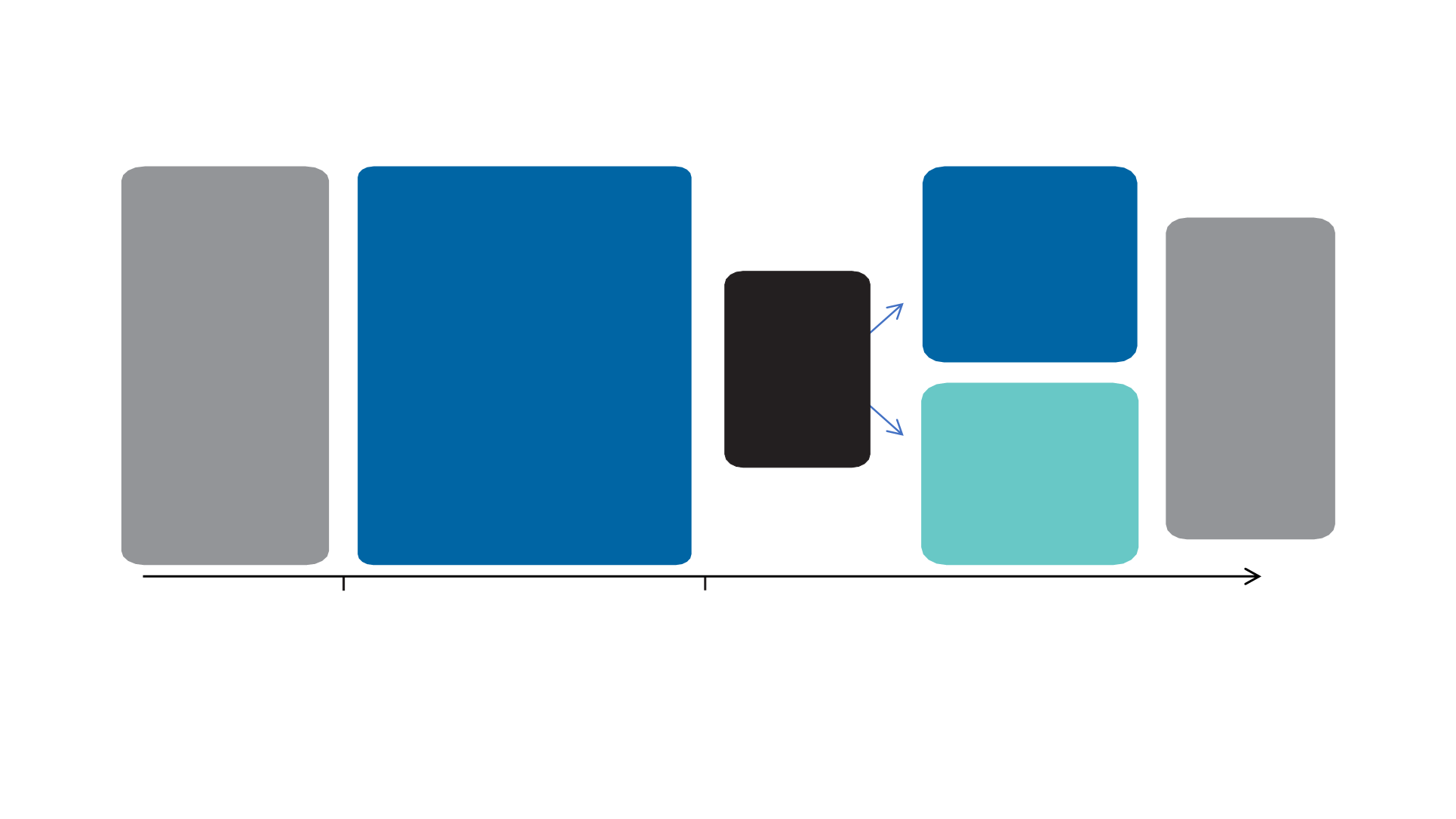
CheckMate 153 Phase IIIB/IV Study Design
a
Conventional systemic therapies, excluding immuno-oncology therapies
CNS = central nervous system; DOR = duration of response; mets = metastases; ORR = objective response rate; PFS = progression-free survival; PK = pharmacokinetics
Screening
study
population
Advanced/
metastatic
(stage IIIB/IV)
NSCLC
(non-SQ and SQ) treated
with
≥1 prior
systemic
therapy
a
Cohort B
Discontinue treatment, and if
progression
occurs, retreatment allowed
Cohort A
Continue to treat
to progression,
unacceptable toxicity, or
withdrawal of informed
consent
Intervention
Nivolumab 3 mg/kg IV Q2W
Patient subgroups
•
SQ, ECOG PS 0-1, ≥2 prior therapies, ± treated
CNS mets
•
SQ, ECOG PS 0-1, 1 prior therapy, ± treated
CNS mets
•
Non-SQ, ECOG PS 0-1,
≥1 prior therapy, ± treated CNS mets
•
SQ or non-SQ, ECOG PS 2, ≥1 prior therapy
Endpoints
•
Safety
•
ORR
•
PFS
•
DOR
•
OS
•
PROs
•
PK
•
Biomarkers
Randomize
1:1
Patients on
treatment at
1 year
Enrollment period:
staggered by
histology,
line of therapy,
and subgroup
Treatment until progression or for 1 year
Follow up for 5 years to collect survival information
Spigel D, et al. WCLC 2016


