*Lymph nodes are identified as target lesion for RECIST evaluation
CR = complete response; NE = not evaluated; PR = partial response; SD = stable disease
31 Jan 2014 cut-off
Please note that alectinib has not yet received regulatory approval in the EU
Tamura, et al. CMSTO 2014; Ohe, et al. ASCO 2015
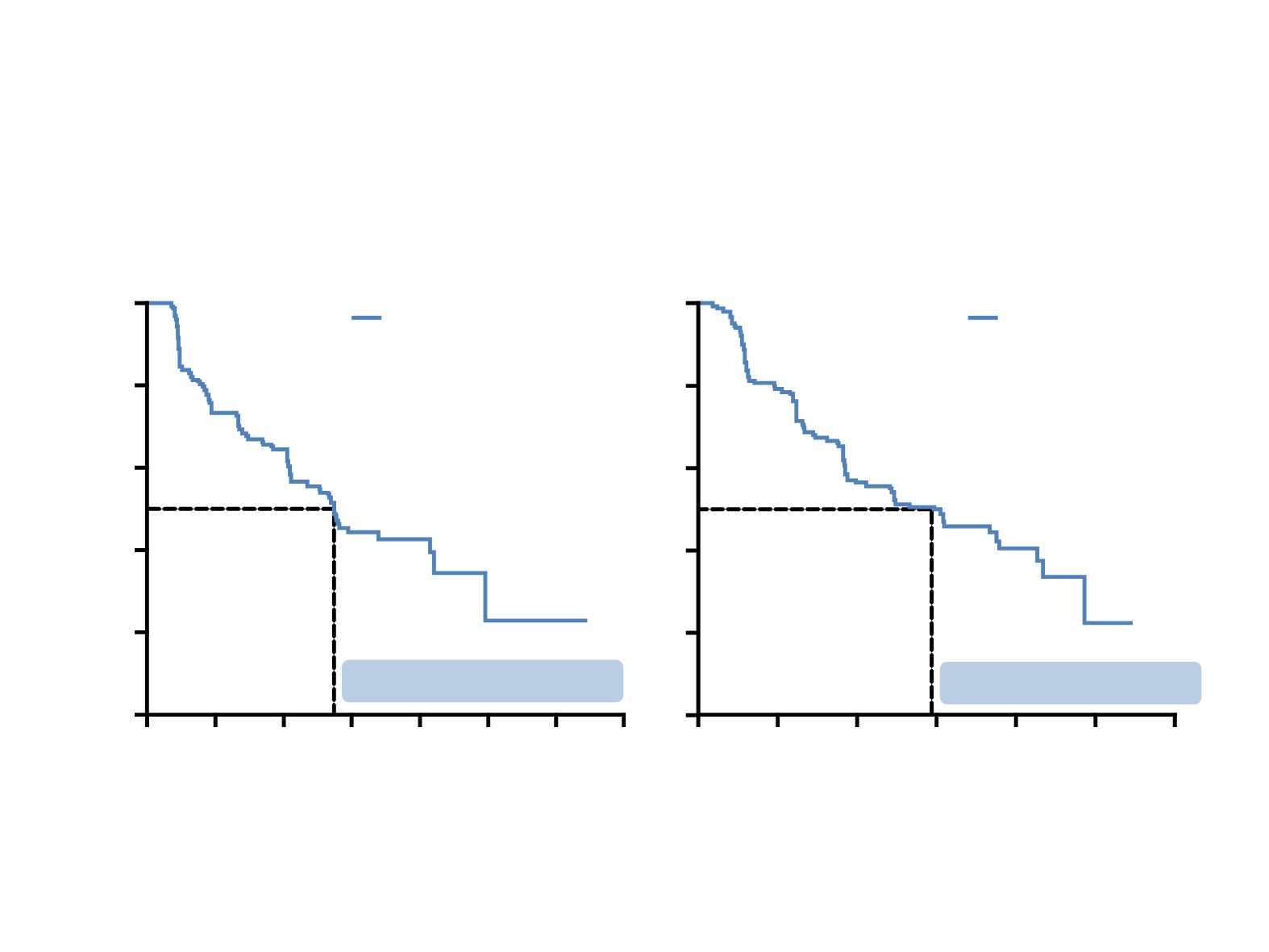
NP28761 and NP28673: PFS (ITT)
Data cut-off NP28761= 27 April 2015; NP28673 = 8 Jan 2015
Please note that alectinib h s not yet received regulatory approval in the EU
Shaw, et al. WCLC 2015; Ou, et al. ASCO 2015
NP28761
NP28673
1.0
0.8
0.6
0.4
0.2
0
PFS estimate
0
3
6
9
Time (months)
12 15 18 21
Median PFS 8.1 months
1.0
0.8
0.6
0.4
0.2
0
0
Time (months)
Alectinib 600mg
(n=138)
18
6
3
9
12
15
Median PFS 8.9 months
Alectinib 600mg BID
(n=87)
INDICACIÓN NO APROBADA, EN INVESTIGACIÓN


