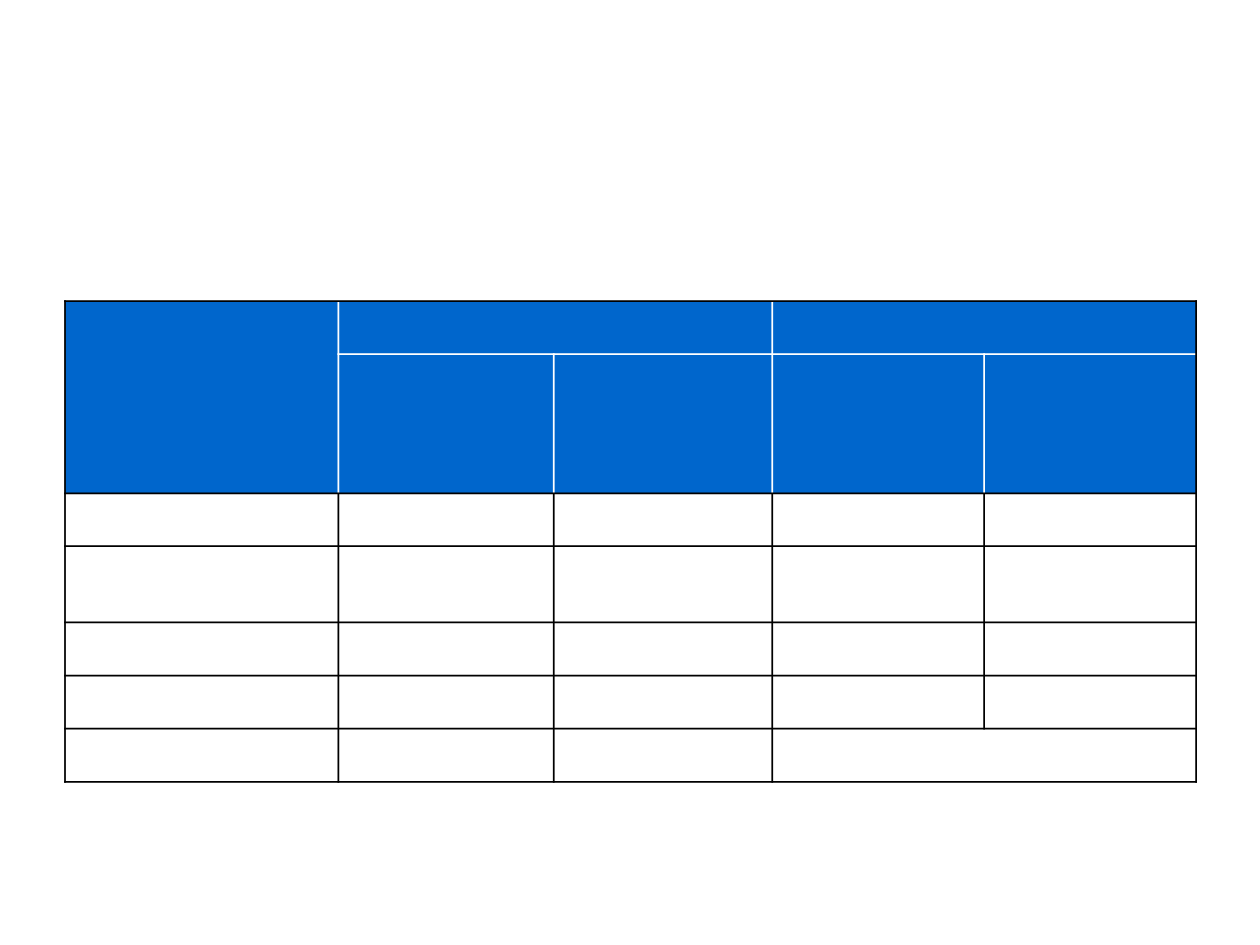
Overview of alectinib in phase II studies of
crizotinib-resistant patients
1. Barlesi, et al. ESMO 2016
2. Shaw, et al. Lancet Oncol 2016; 3. Gadgeel, et al. J Clin Oncol 2016
Systemic efficacy
Intracranial efficacy: pooled analysis
3
Global
1
North American
2
Measurable
and/or non-
measurable CNS
disease
Measurable CNS
disease
Number of patients
138
87
136
50
Objective response
rate, %
51
52
43
64
Median PFS, months
8.9
8.1
–
–
Median DoR, months
15.2
13.5
11.1
10.8
Data cut-off
1 February 2016
27 April 2015
27 April 2015
Global study safety summary
1
•
Most common AEs: constipation (38%), fatigue (31%) and peripheral oedema (30%)
•
Dose reductions reported in 11% of patients, and withdrawals in 9%
INDICACIÓN NO APROBADA, EN INVESTIGACIÓN


