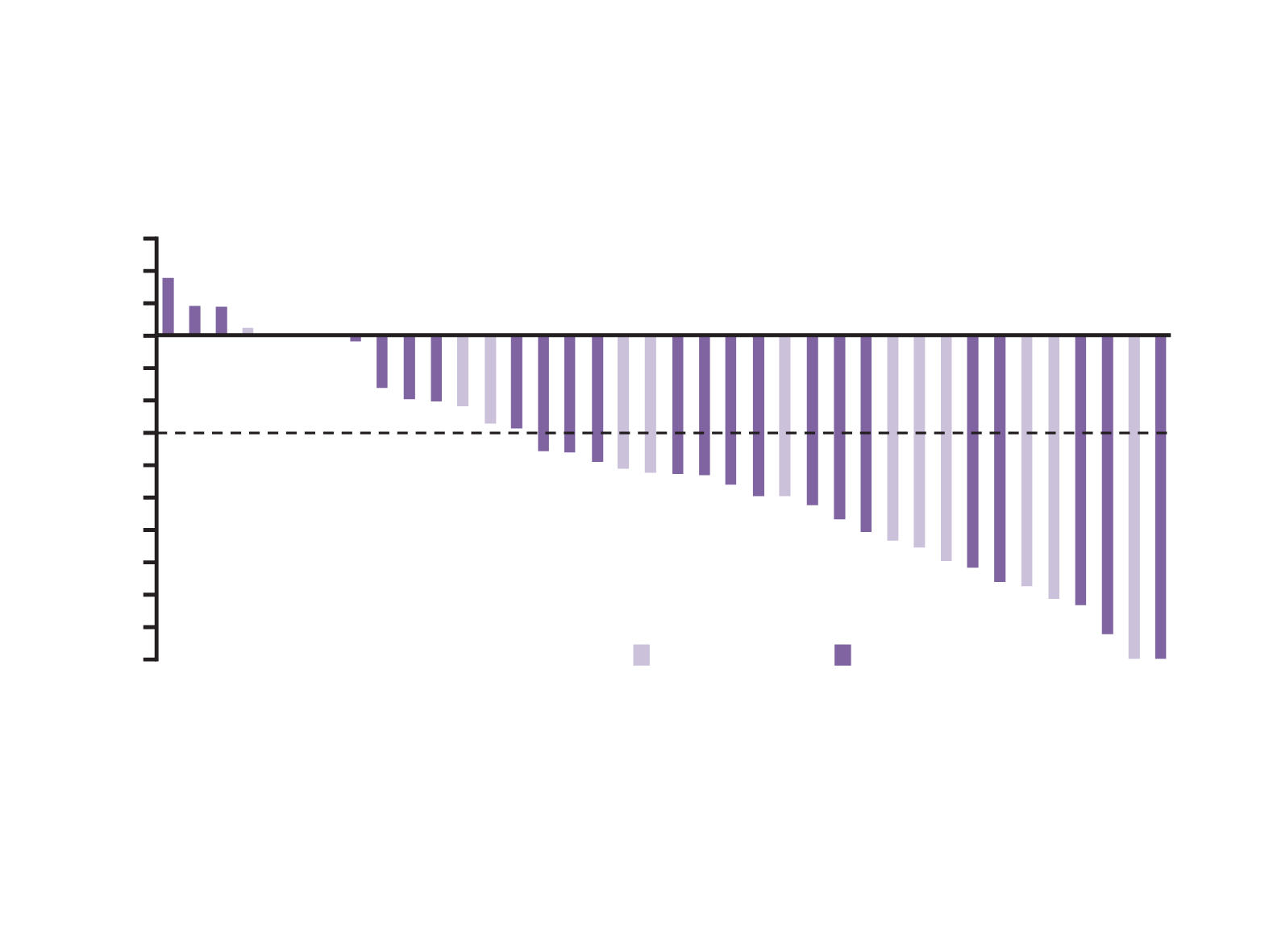
Antitumour activity and safety of lorlatinib in
advanced
ALK
+ NSCLC
*Ongoing treatment
Two patients with indeterminate responses excluded
Number of prior TKIs counted by line; patients may have received the same drug more than once, either sequentially or separated by another therapy
Solomon, et al. ASCO 2016
*
*
30
-100
-90
-80
-70
-60
-50
-40
-30
-20
-10
0
10
20
Best change from baseline (%)
1 prior TKI
≥2 prior TKI
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
•
Most common AEs: hypercholesterolaemia (82%), peripheral oedema (53%) and hypertriglyceridaemia (41%)
•
Lorlartinib is also associated with neurological adverse events, such as slow speech (18% of patients)
INDICACIÓN NO APROBADA, EN INVESTIGACIÓN


