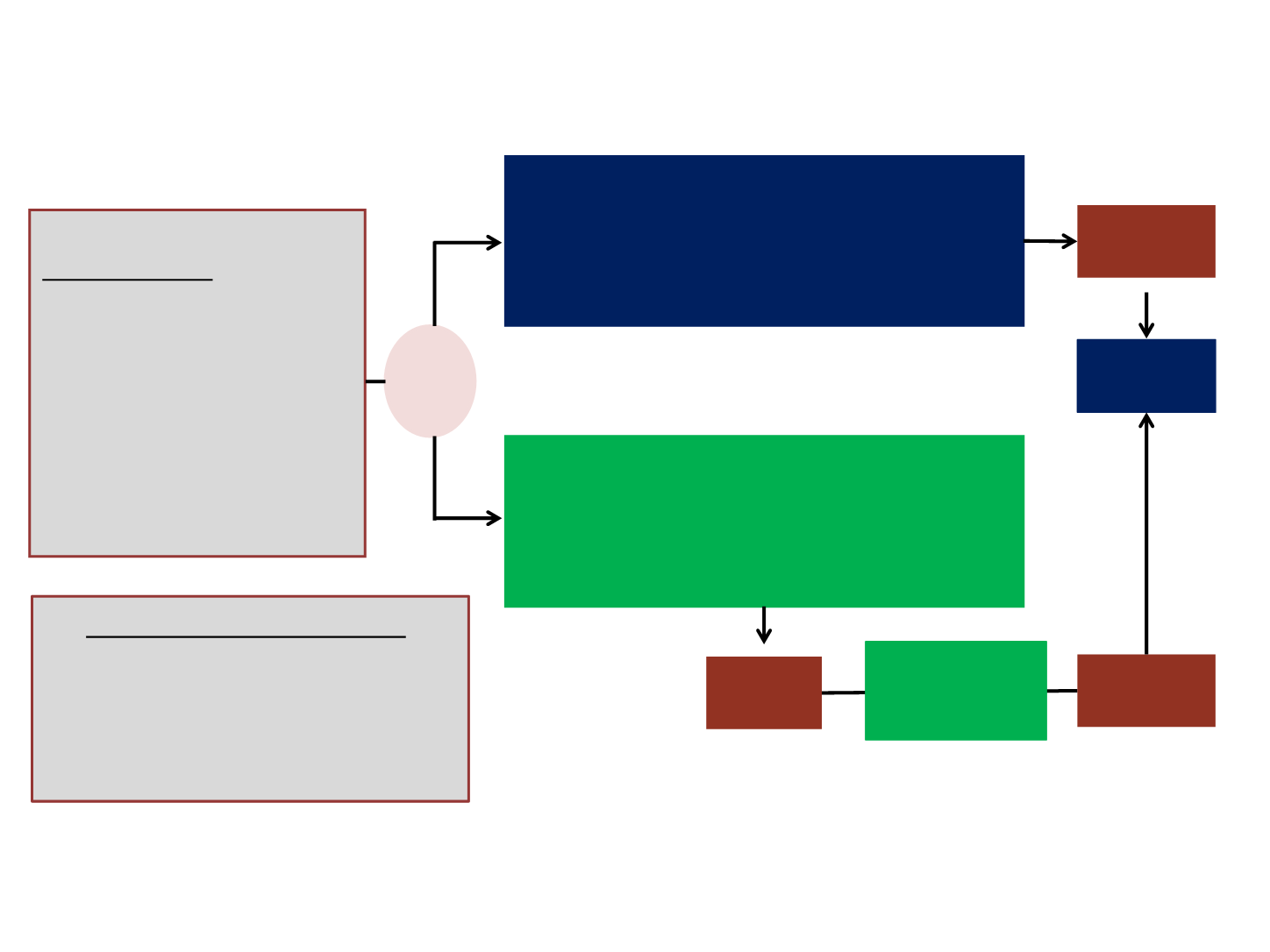
ASCEND 4 - Phase 3, Randomized, Global, Open-label Study
(NCT01828099)
†
One cycle = 21 days
BIRC, Blinded Independent Review Committee; CR, complete response; IHC, immunohistochemistry; PD, progressive disease; PR; partial response; PS, performance
status; SD, stable disease; WHO, World Health Organization;
Inclusion criteria
•
Stage IIIB/IV
ALK
+ NSCLC by
Ventana IHC test (central)
•
Treatment-naive (no prior
chemotherapy or ALK
inhibitor)
•
WHO PS 0-2
•
Neurologically stable brain
metastases (symptomatic or
not)
Optional
Pemetrexed
maintenance
500 mg/m
2
q21d
CR, PR,
SD
Chemotherapy (Induction Investigator choice)*:
Four cycles
†
Pemetrexed 500 mg/m
2
+ cisplatin 75 mg/m
2
or
Pemetrexed 500 mg/m
2
+ carboplatin AUC 5–6
Optional
crossover
to
extension
treatment
Ceritinib 750 mg/day
†
•
Daily oral dosing in fasted state
PD (BIRC
confirmed)
Ceritinib
750 mg
R
1:1
Stratified randomization:
WHO PS
Brain metastases
Prior neoadjuvant/adjuvant
chemotherapy
*At the time when ASCEND-4 was designed and initiated, pemetrexed-platinum chemotherapy followed by pemetrexed maintenance
was the standard of care in patients with non-squamous advanced NSCLC
PD (BIRC
confirmed)


