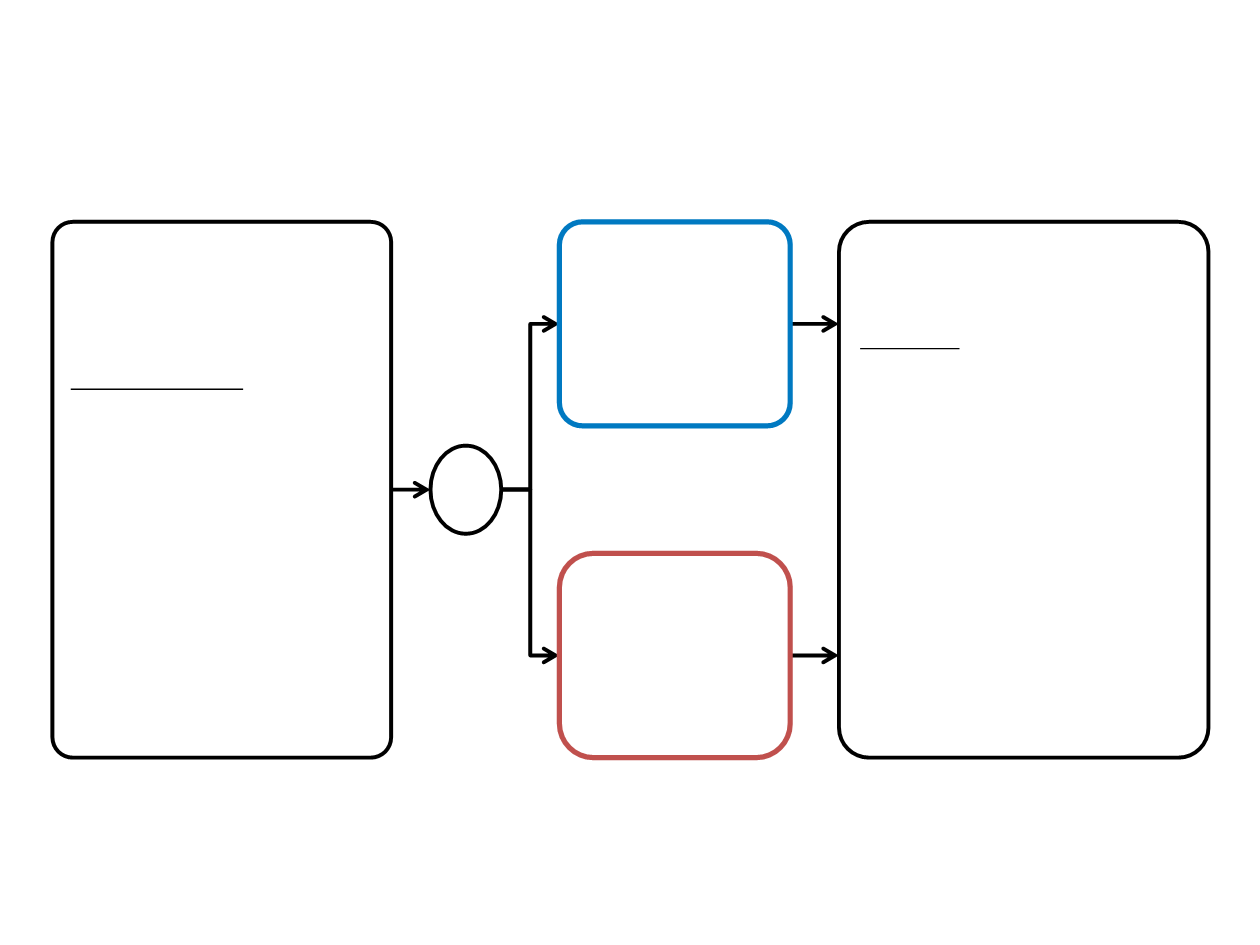
J-ALEX: phase III study design
Key Entry Criteria
•
Stage IIIB/IV or recurrent
ALK-positive NSCLC
•
ALK centralized testing
•
ECOG PS 0-2
•
Treated/asymptomatic
brain metastases allowed
•
≤1 prior chemotherapy
R
1:1
Endpoints
•
Primary
•
PFS
•
Secondary
•
OS
•
ORR
•
PK
•
QOL
•
CNS PFS
•
Safety
Alectinib 300
mg
BID PO
(n=100)
Crizotinib 250 mg
BID PO
(n=100)
Nokihara, et al, ASCO 2016 (Abs. 9008)
INDICACIÓN NO APROBADA, EN INVESTIGACIÓN


