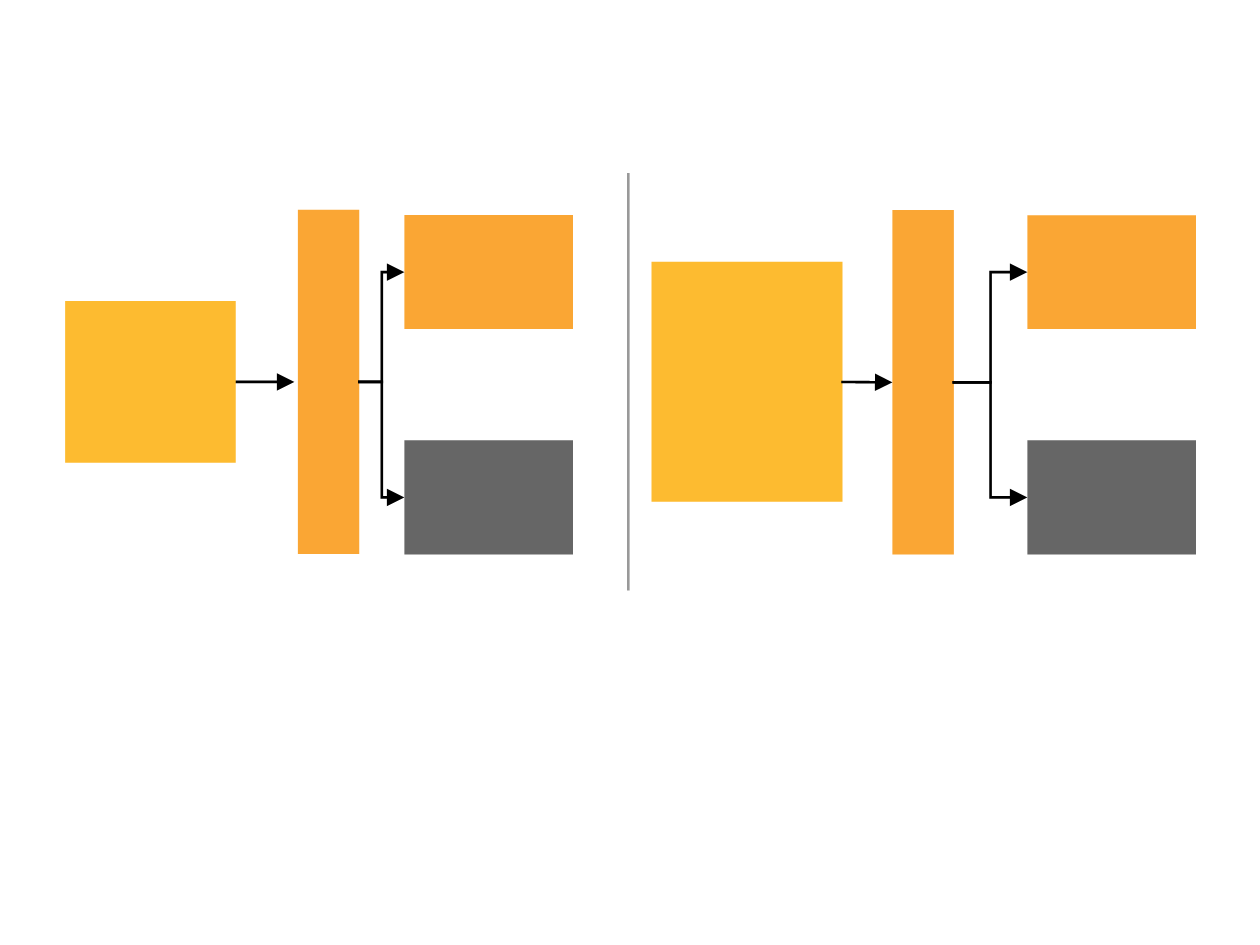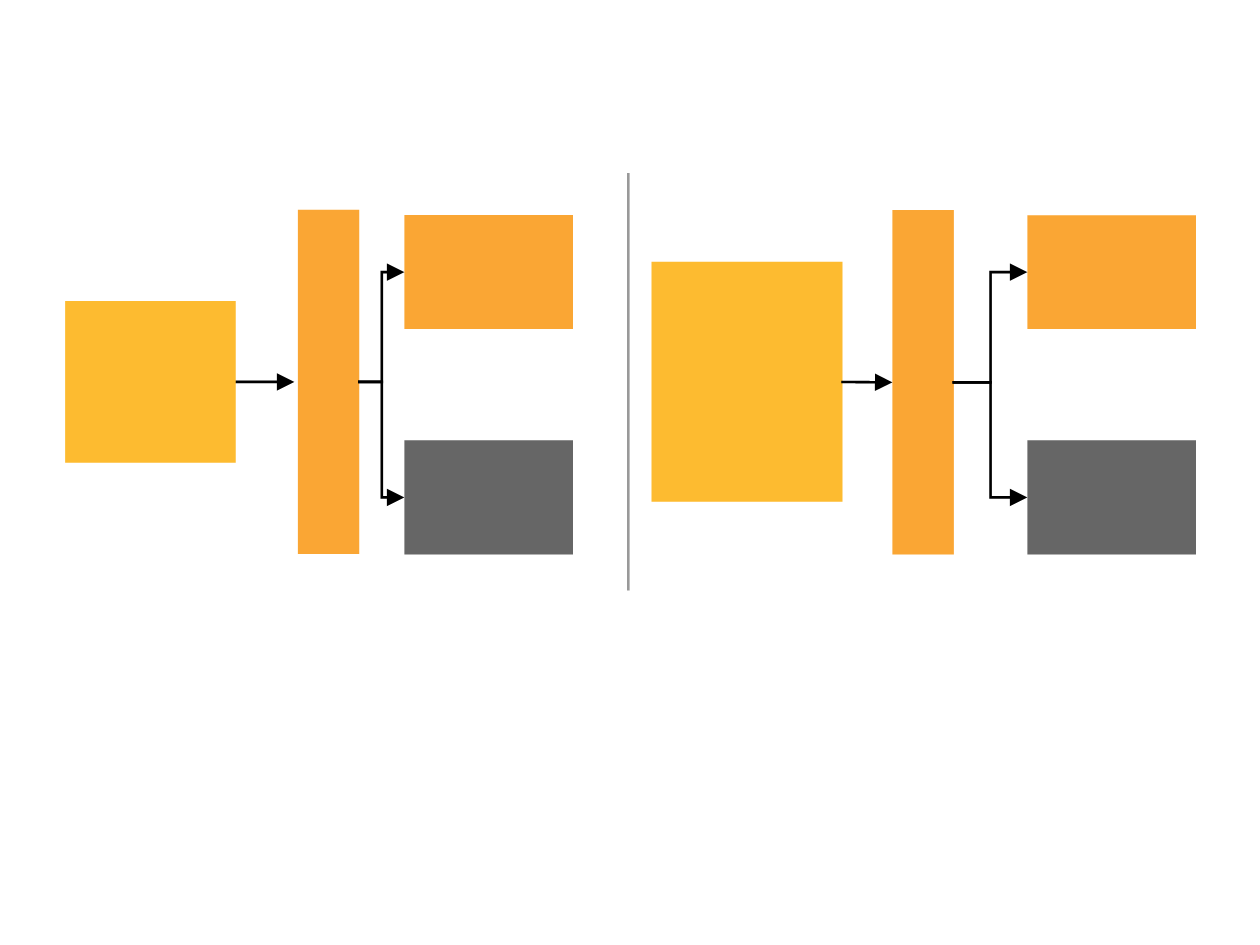
Randomised Phase II open-label trial at 50 centres in 12 countries (NCT00721409)
Key eligibility criteria: inoperable locally recurrent disease, postmenopausal status, no prior
therapy for advanced breast cancer, no prior CDK inhibitors, no letrozole within 12 months,
no prior/current brain metastases, measurable disease (RECIST 1.0) or bone-only disease,
ECOG PS ≤1, adequate bone marrow and renal function
PALOMA-1: Study design
*Randomisation stratified by disease site and disease-free interval
†
Palbociclib schedule 3/1 (28-day cycles)
Finn RS, et al. Lancet Oncol 2015;16:25–35
n=66
1:1
Cohort 1
Cohort 2
n=99
1:1
ER+ HER2–
advanced breast
cancer
ER+ HER2–
advanced breast
cancer
with
CCND1
amplification
and/or loss of p16
R
A
N
D
O
M
I
S
A
T
I
O
N
*
R
A
N
D
O
M
I
S
A
T
I
O
N
*
Palbociclib 125
mg/day
†
+
letrozole
2.5 mg/d
Palbociclib 125
mg/day
†
+
letrozole
2.5 mg/d
Letrozole
2.5 mg/d
Letrozole
2.5 mg/d