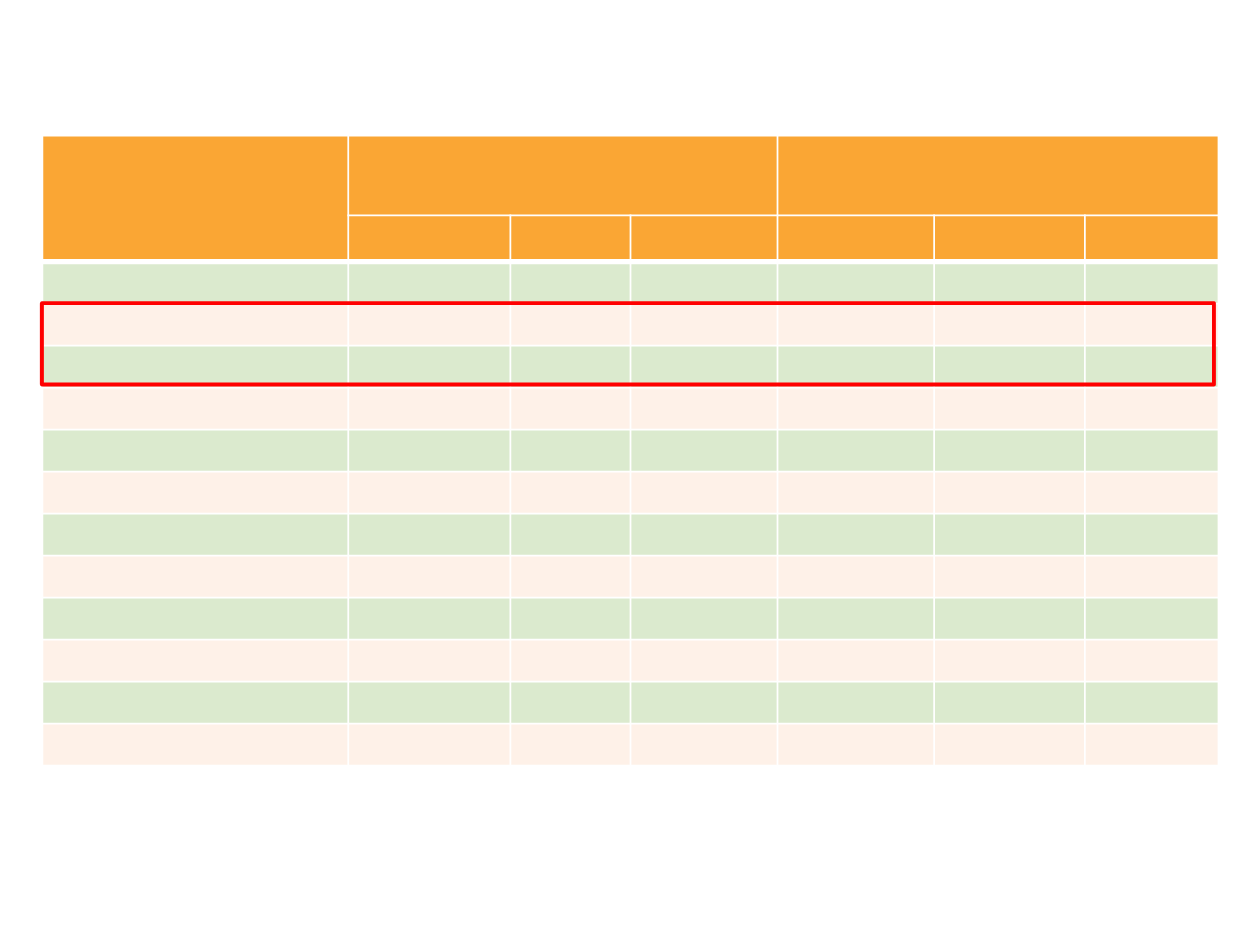
AE, %
Palbociclib* + Fulvestrant
(n = 345)
Placebo + Fulvestrant
(n = 172)
Any Grade Grade 3
Grade 4
Any Grade
Grade 3
Grade 4
Any AE
98
59
11
89
16
2
Neutropenia
79
53
9
4
0
1
Leukopenia
46
25
1
4
0
1
Anaemia
26
3
0
10
2
0
Thrombocytopenia
19
2
1
0
0
0
Fatigue
38
2
0
27
1
0
Nausea
29
0
0
26
1
0
Headache
21
<1
0
17
0
0
Upper respiratory infection
a
19
<1
0
16
0
0
Diarrhoea
19
0
0
17
1
0
Constipation
17
0
0
14
0
0
Alopecia
15
0
0
6
0
0
Phase III PALOMA-3 Adverse Events
AE, adverse event. AEs with ≥15% incidence in the palbociclib + fulvestrant group reported.
a
Upper respiratory infection includes influenza, influenza-like illness, laryngitis, nasopharyngitis or pharyngitis, rhinitis, sinusitis, and
upper respiratory tract infection. *Investigational drug
.
Turner NC, et al. N Engl J Med. 2015


