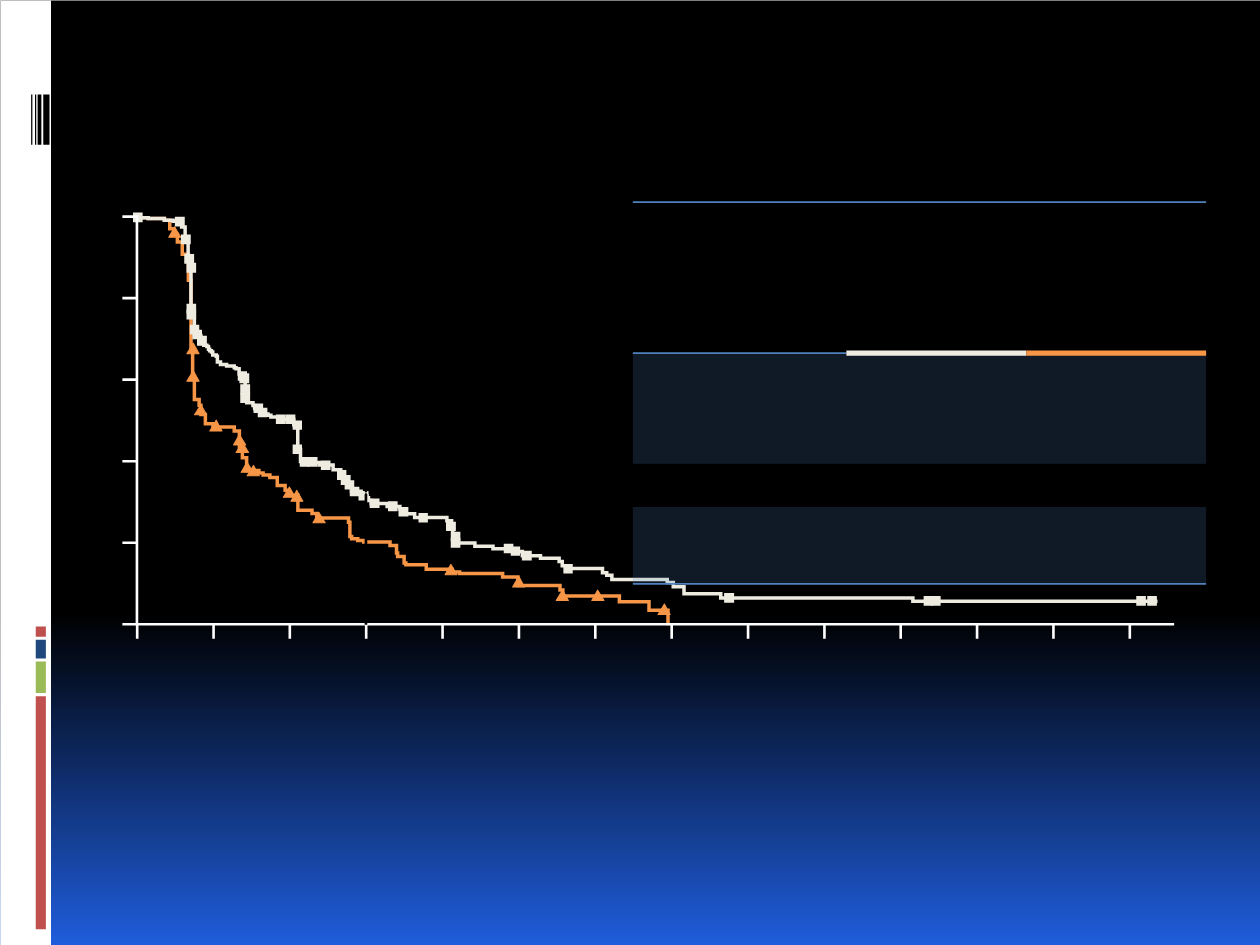
PFS results by independent central review were consistent with local assessment:
HR 0.57 (95%CI: 0.44–0.74; one-sided p<0.001)
Progression-free Survival per Investigator
Assessment
(Primary Endpoint)
CI, confidence interval; HR, hazard ratio.
6-month PFS rate:
31% vs. 20%
100
80
60
40
20
0
0
4
8
2
6
10
12
14
16
18
20
22
24
26
Time, Months
Probability of
Progression-free Survival, %
Full
Population
(N=432)
Buparlisib
+
Fulvestrant
n=289
Placebo +
Fulvestrant
n=143
Median PFS,
months (95%
CI)
3.9
(2.8–4.2)
1.8
(1.5–2.8)
HR (95% CI)
0.67 (0.53–0.84)
One-sided p-
value
<0.001


