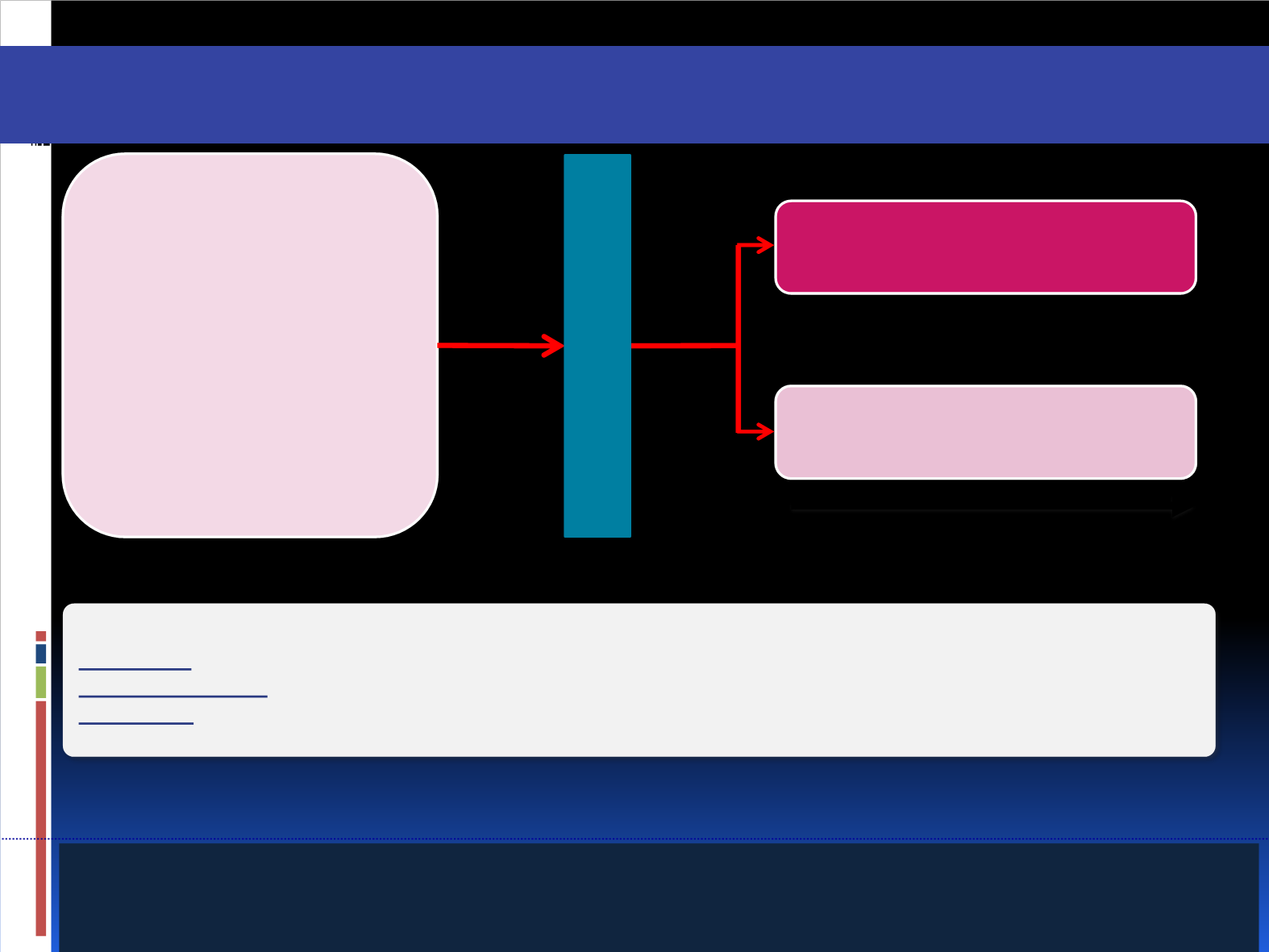
BELLE-3: Fulvestrant
±
Buparlisib Phase III Study in
HR+/HER2– ABC: AI Pretreated and mTORi Resistant
Stratification by visceral disease
Study objectives:
Co-primary:
PFS (local assessment)
Co-key secondary:
OS
Secondary:
Efficacy (ORR, CBR), safety, PK, patient-reported outcomes
,
time to ECOG PS deterioration
Fulvestrant + placebo
(n=140)
Fulvestrant + buparlisib
(n=280)
N≈420
•
Locally advanced or metastatic
HR+/HER2– BC
•
Postmenopausal
•
Pretreated with an aromatase inhibitor
•
≤1 previous chemotherapy for mBC
•
Refractory to mTOR-inhibitor/
endocrine combination therapy
•
ECOG PS ≤2
NCT01633060/CBKM120F2303.
1. BELLE-3 Study Protocol, version no. 02; 19-Dec-2013. NCT01633060 CBKM120F2303
Therapy until disease
progression, intolerable toxicity,
or withdrawal of consent
R
A
N
D
O
M
I
Z
E
2:1


