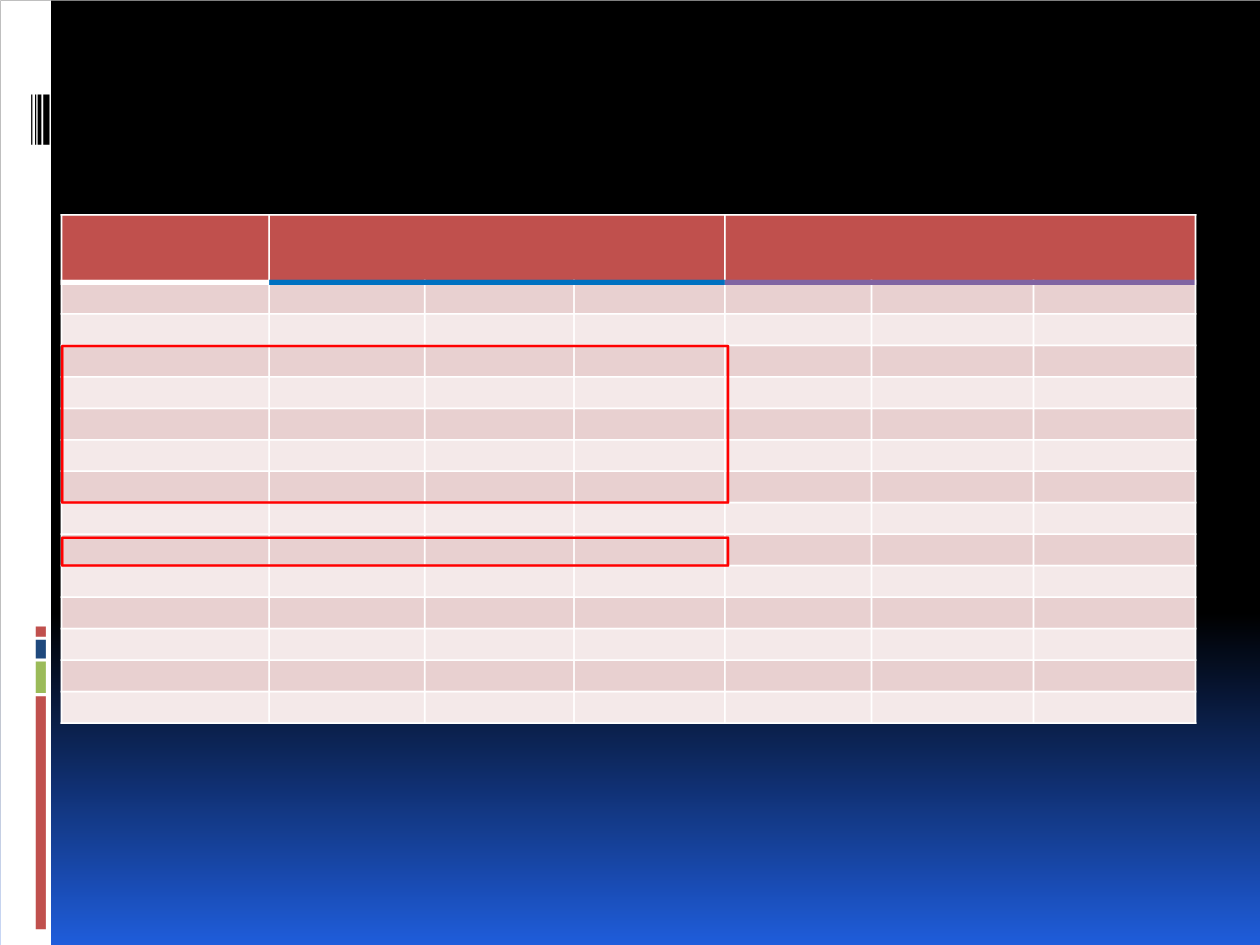
Serious adverse events occurred in 23.4% of patients in the buparlisib arm vs 15.8% in the placebo arm
12 on-treatment deaths (2.1%) were reported in each arm in the full population, the majority due to disease
progression
Buparlisib + Fulvestrant
n=573
Placebo + Fulvestrant
n=570
Adverse event, %
All grades
Grade 3
Grade 4
All grades
Grade 3
Grade 4
Total
99.5
63.2
14.1
93.0
27.4
4.6
Increased ALT
40.1
18.7
6.8
6.8
1.1
0
Increased AST
37.3
15.0
3.0
9.3
2.8
0
Hyperglycemia
43.1
15.2
0.2
7.7
0.2
0
Rash
32.1
7.7
0.2
6.3
0
0
Anxiety
22.3
5.2
0.2
8.2
0.9
0
Fatigue
31.9
4.9
0
23.9
1.6
0
Depression
26.2
3.7
0.7
8.9
0.4
0
Diarrhea
34.2
3.7
0
14.6
1.1
0
Asthenia
20.1
2.8
0
10.5
1.1
0
Stomatitis
21.6
2.1
0
6.5
0.5
0
Nausea
38.7
1.7
0
23.2
1.4
0
Decreased appetite
29.8
1.6
0
11.1
0.2
0
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
1
6
0
9
0
4
3
6
8
1
toxicidad


