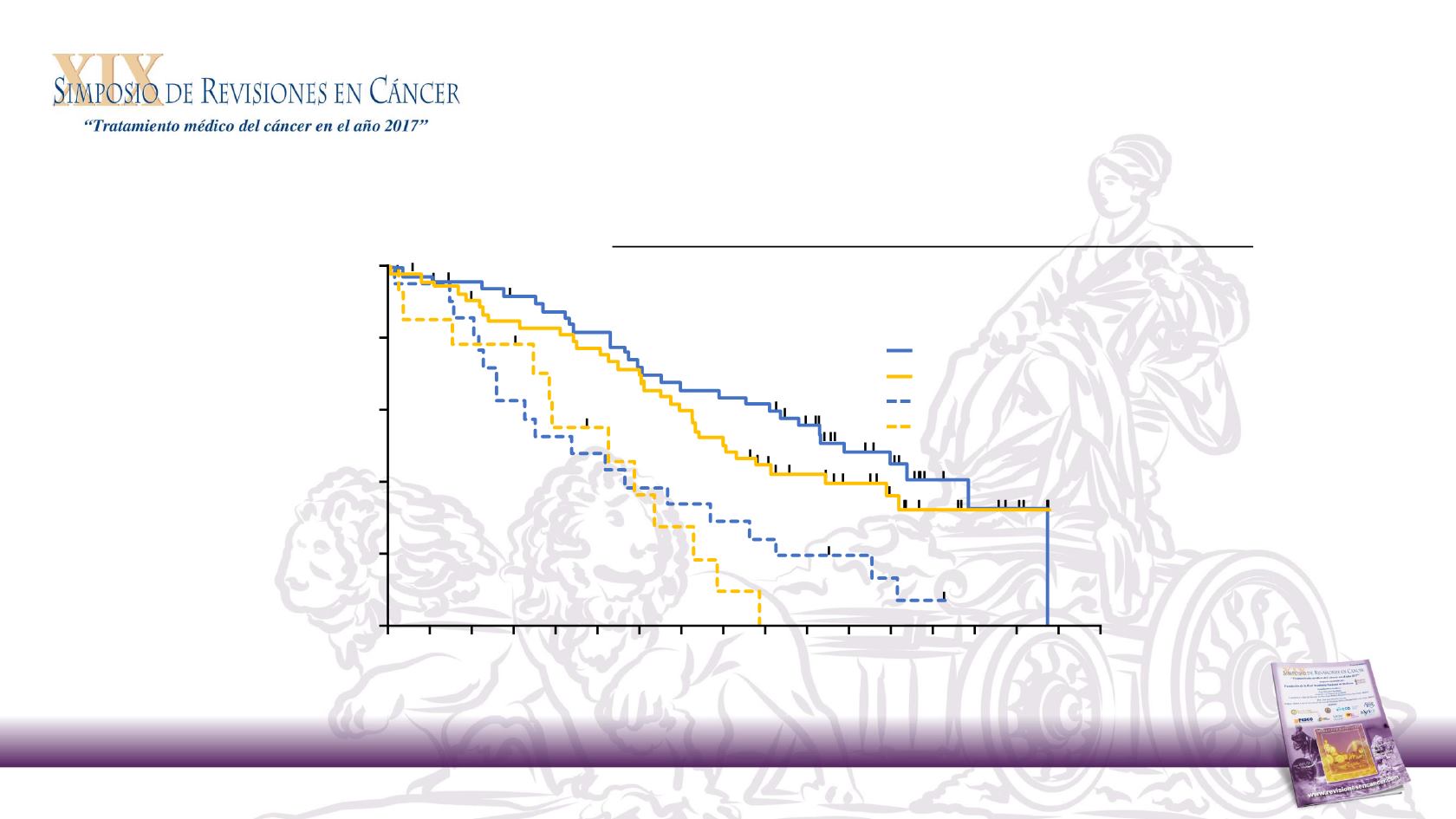
Panitumumab outcome by tumour location analysis
Phase 2 PEAK study: OS
•
Boeckx N, et al. Ann Oncol 2016;27(Suppl 6):abstract 89P (and poster).
•
†
Updated values will be included in the full publication. NA, not available.
68
64
60
56
52
48
44
40
36
32
28
24
20
16
12
8
4
0
0
20
40
60
80
100
Kaplan–Meier estimate
Months
0
0
2
2
3
3
0
6
5
1
13
10
2
17
13
3
25
17
4
31
21
5
0
32
26
6
1
33
31
7
3
36
35
8
4
41
40
10
6
44
43
11
7
46
44
13
11
49
48
18
11
51
51
21
12
53
54
22
14
1:
2:
3:
4:
No. of subjects
3: Pmab + FOLFOX right side
1: Pmab + FOLFOX left side
2: Bev + FOLFOX left side
4: Bev + FOLFOX right side
Median OS, months
Pmab + FOLFOX
Bev + FOLFOX
HR (95% CI)
†
Left
43.4
32.0
NA
Right
17.5
21.0
NA
WT
RAS


