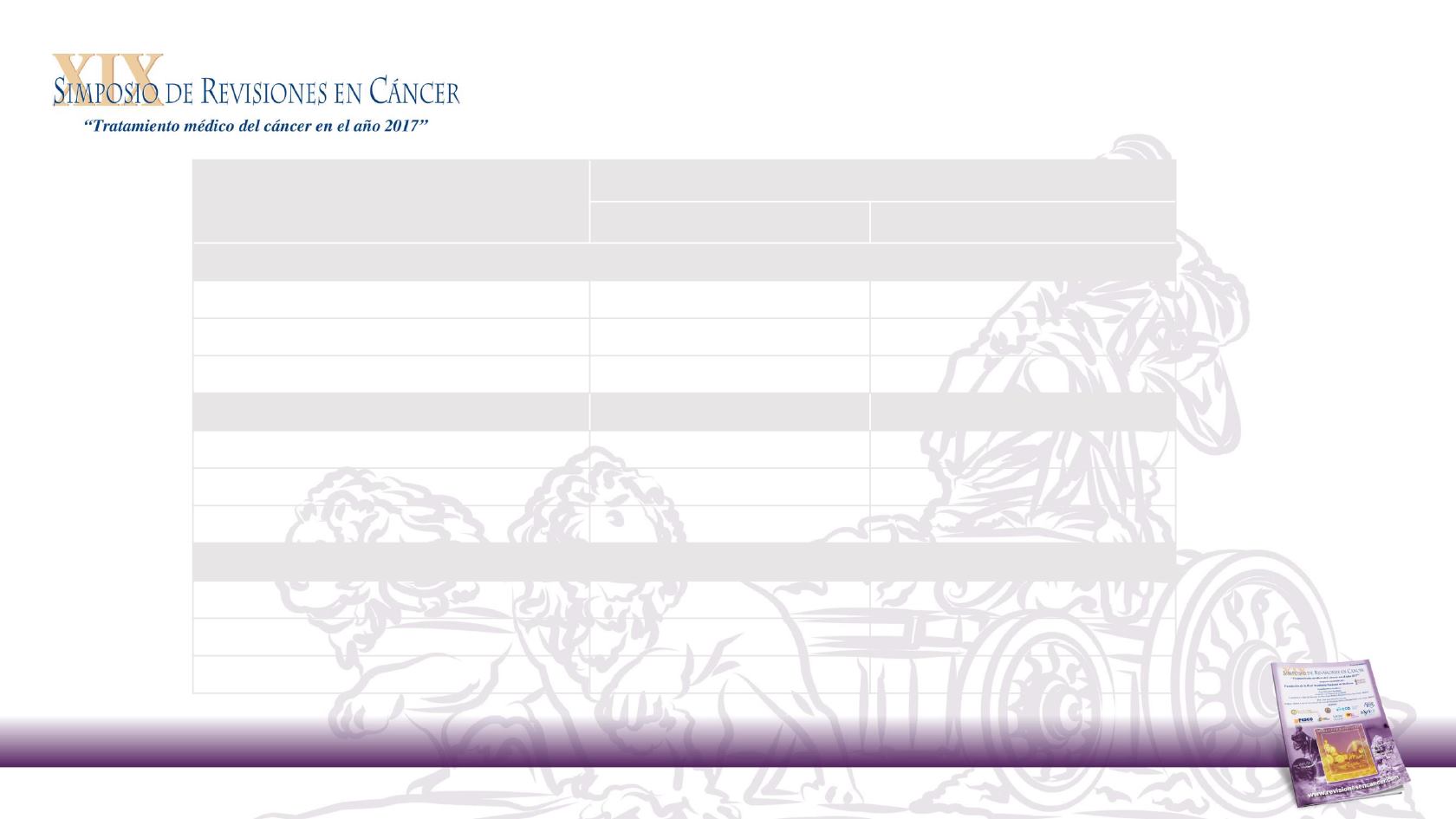
> 80% ascertainment for tumour side (blinded to treatment outcome)
†
WT
RAS
Primary tumour location, %
Left
Right
PRIME (n = 512)
†
All evaluable pts (n = 416)
79
21
Pmab + FOLFOX (n = 208)
81
19
FOLFOX (n = 208)
76
24
PEAK (n = 170)
†
All evaluable pts (n = 143)
75
25
Pmab + FOLFOX (n = 75)
71
29
Bev FOLFOX (n = 68)
79
21
Study 181 (n = 421)
†
All evaluable patients (n = 368)
81
19
Pmab + FOLFIRI (n = 181)
83
17
FOLFIRI (n = 187)
79
21
Panitumumab outcome by tumour location analysis
Primary tumour
location in PRIME, PEAK and
Study 181
•
Boeckx N, et al. Ann Oncol 2016;27(Suppl 6):abstract 89P (and
poster).
•
†
Tumour side ascertainment: PRIME, 81%; PEAK, 84%; Study 181, 87%.
Pmab, panitumumab; bev, bevacizumab.


