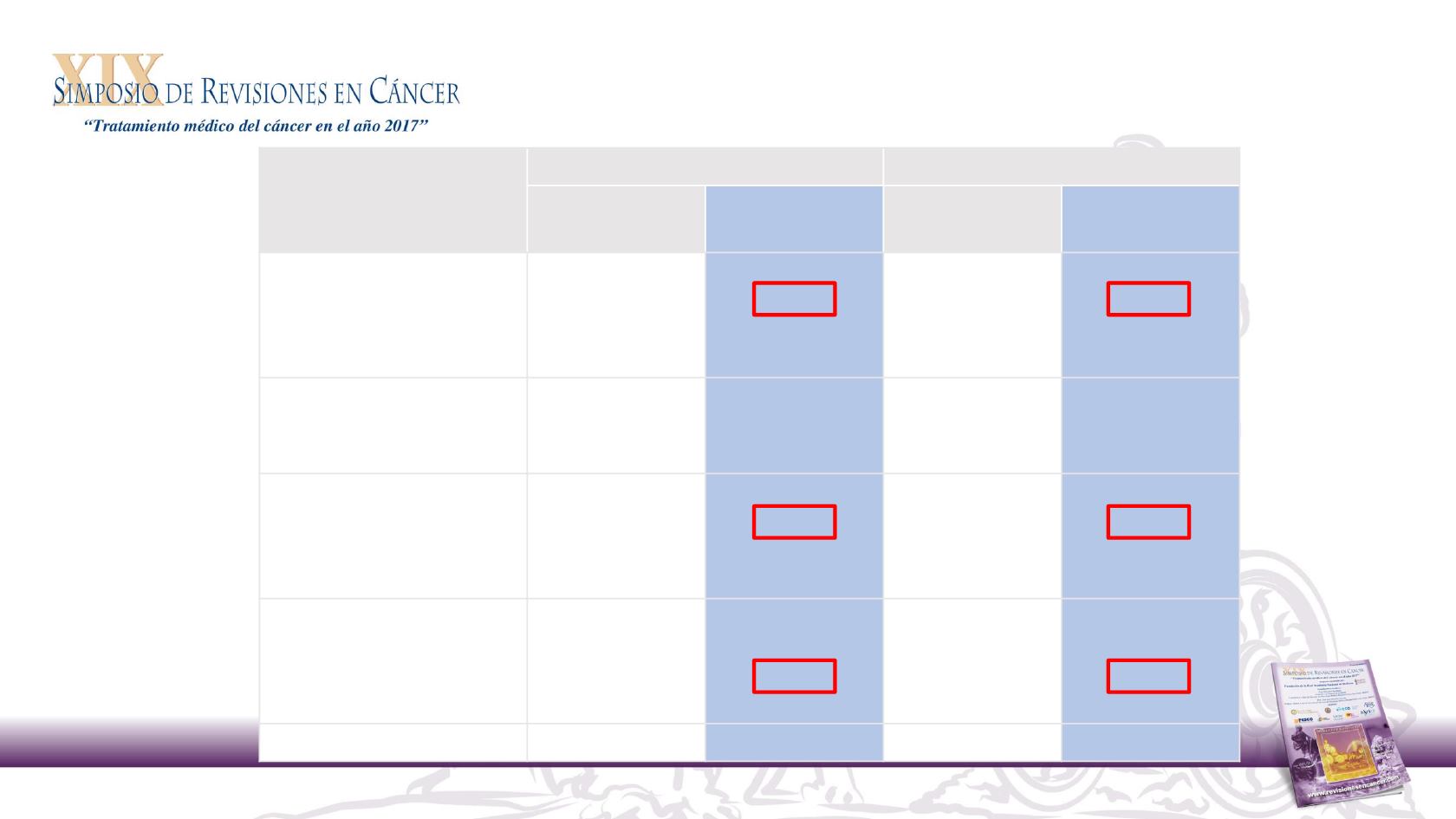
Panitumumab outcome by tumour location analysis
Phase 2 PEAK study: baseline characteristics
•
Boeckx N, et al. Ann Oncol 2016;27(Suppl 6):abstract 89P (and poster).
WT
RAS
Panitumumab + FOLFOX
Bevacizumab + FOLFOX
Left
(n = 53)
Right
(n = 22)
Left
(n = 54)
Right
(n = 14)
ECOG PS, %
0
1
2
69.8
30.2
-
45.5
54.5
-
64.8
35.2
-
64.3
35.7
-
Prior adjuvant, %
No
Yes
84.9
15.1
81.8
18.2
75.9
24.1
71.4
28.6
BRAF
status, %
Mutant
Wild type
Failure
1.9
98.1
-
40.9
59.1
-
1.9
98.1
-
7.1
92.9
-
Metastatic sites, %
Liver + other
Liver only
Other only
39.6
34.0
26.4
59.1
18.2
22.7
38.9
27.8
33.3
64.3
28.6
7.1
Median age, years
60
64
60
66


