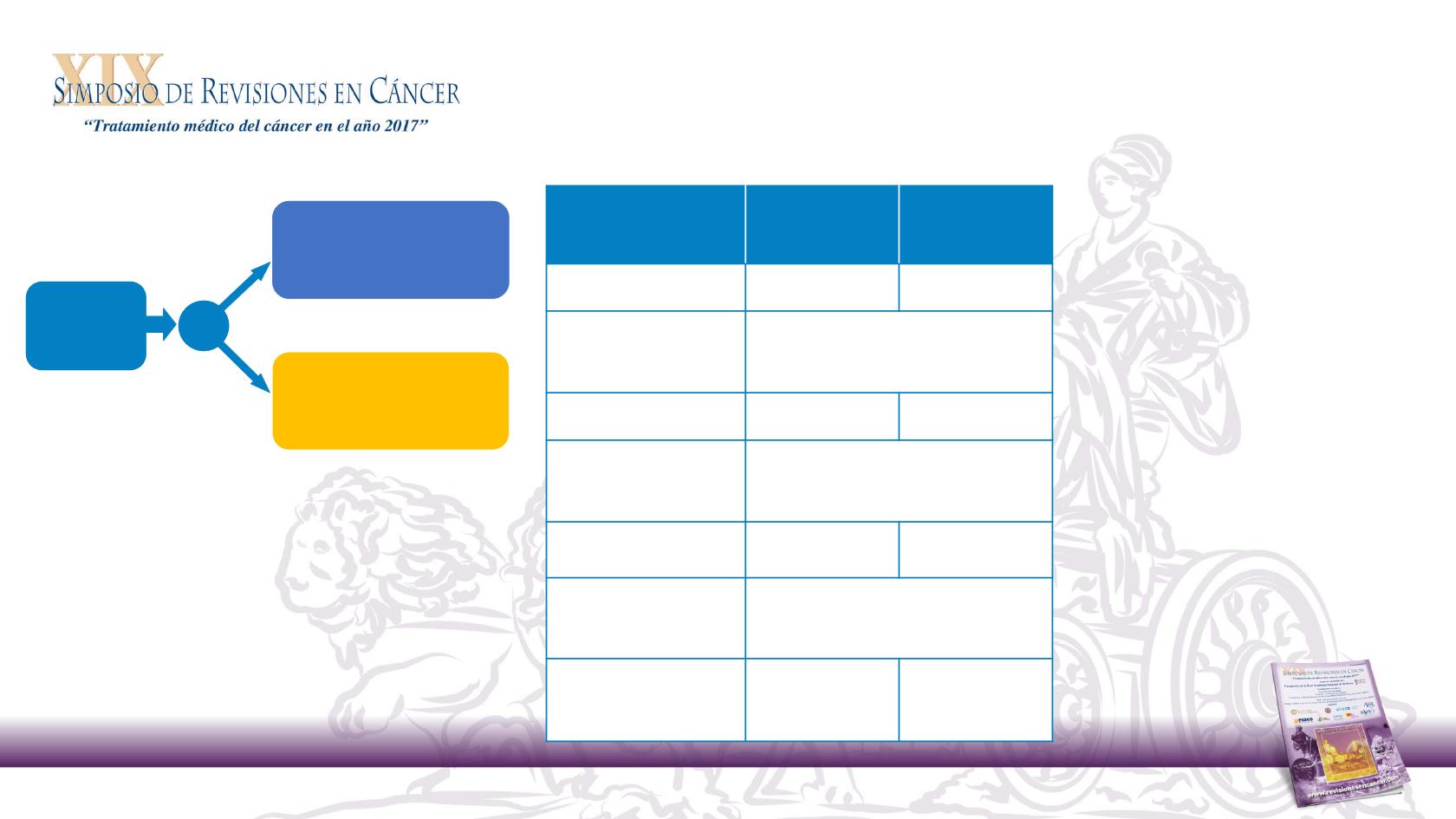
Phase 2 PEAK study overview
mFOLFOX6 + panitumumab or bevacizumab in
1
st
-line treatment of mCRC
•
1. Schwartzberg LS, et al. J Clin Oncol 2014;32:2240−7;, 2. Rivera F, et al. Eur J Cancer 2015;51(Suppl
3):S1‒S810:abstract 2014 (and poster).
•
†
Final analysis.
WT
RAS
= WT
KRAS
and
NRAS
exons 2, 3, 4.
Primary endpoint: PFS
1
No planned formal
hypothesis testing
Prespecified extended
RAS
analysis
1,2
RAS
ascertainment rate: 82%
WT
RAS
Panitumumab
+ mFOLFOX6
(n = 88)
Bevacizumab
+ mFOLFOX6
(n = 82)
Median PFS, mo
†2
12.8
10.1
HR
(95% CI)
P-value
0.68
(0.48–0.96)
P = 0.029
Median OS, mo
†2
36.9
28.9
HR
(95% CI)
P-value
0.76
(0.53–1.11)
P = 0.15
ORR, %
†2
(n = 88)
65
(n = 81)
60
OR
(95% CI)
P-value
1.12
(0.56‒2.22)
P = 0.86
AEs, %
1
Grade 3/4
Grade 5
(n = 86)
89.5
4.7
(n = 80)
72.5
8.8
mCRC
WT
KRAS
1
(N = 285)
R
Panitumumab 6 mg/kg
(Q2W)
+
mFOLFOX6
(Q2W)
Bevacizumab 5 mg/kg
(Q2W)
+
mFOLFOX6
(Q2W)


