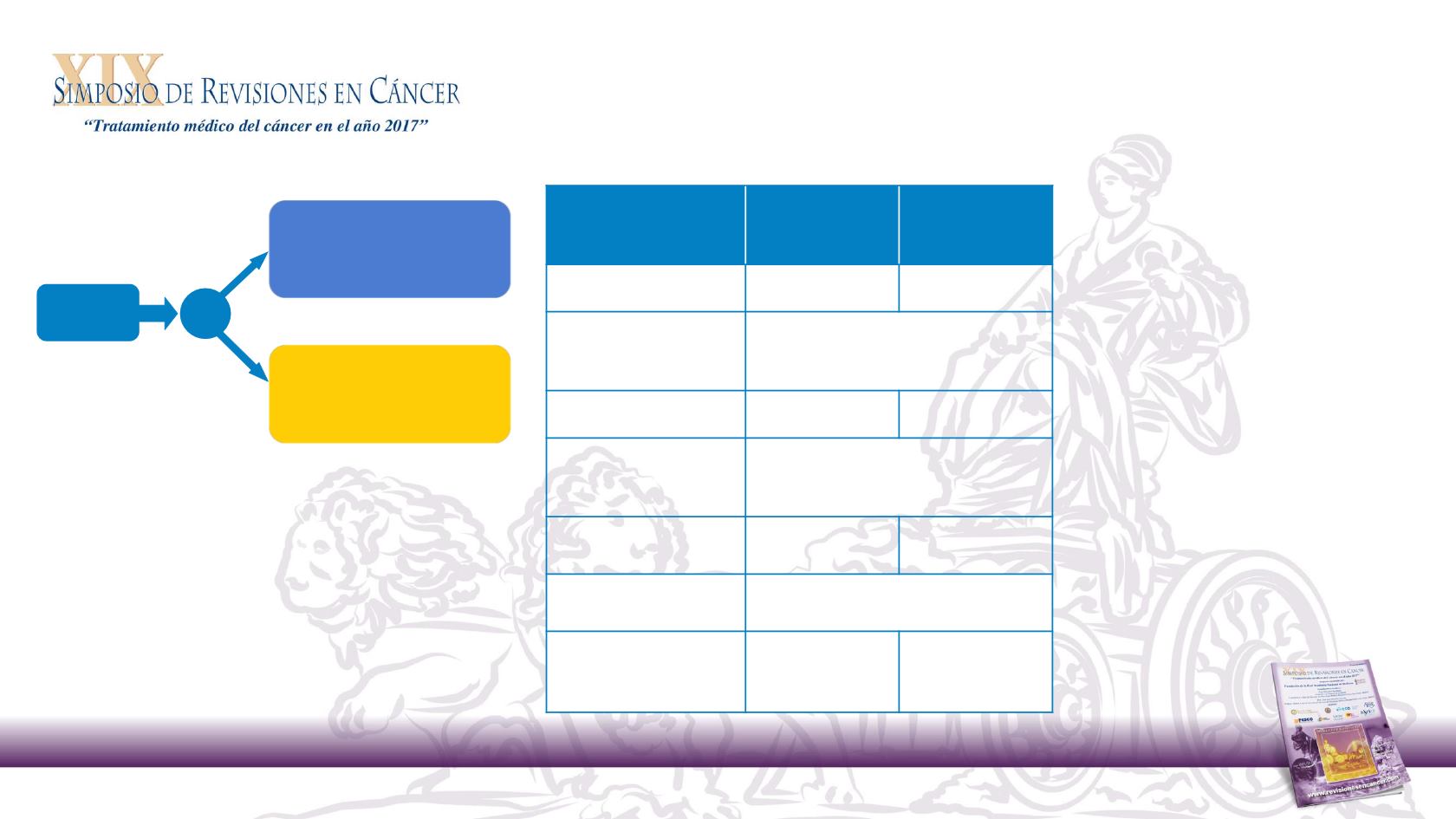
PRIME study overview
FOLFOX4 ± panitumumab in 1
st
-line
Treatment of mCRC
•
1. Douillard JY, et al. J Clin Oncol 2010;28:4697−705;
2. Douillard JY, et al. N Engl J Med 2013;369:1023−34;
•
*Design amended to focus on prospective hypothesis testing in the WT
KRAS
(codons 12 and 13) stratum;
†
Primary analysis;
‡
By central radiological assessment. WT
RAS
= WT
KRAS
and
NRAS
exons 2, 3, 4
•
Primary endpoint: PFS
1
•
Prospective−retrospective
extended
RAS
analysis
2
–
RAS
ascertainment rate: 90%
1:1
FOLFOX4
(Q2W)
+
panitumumab 6 mg/kg
(Q2W)
FOLFOX4
(Q2W)
mCRC*
1
(N = 1183)
WT
RAS
Panitumumab +
FOLFOX4
(n = 259)
FOLFOX4
(n = 253)
Median PFS, mo
†2
10.1
7.9
HR
(95% CI)
P-value
0.72
(0.58–0.90)
P = 0.004
Median OS, mo
†2
26.0
20.2
HR
(95% CI)
P-value
0.78
(0.62–0.99)
P = 0.04
ORR, n (%)
†‡3
(95% CI)
149 (59)
(52–65)
114 (46)
(40–53)
Adjusted OR
P-value
1.63
P = 0.009
AE, %
2
Grade 3/4
Grade 5
(n = 256)
84.8
5.5
(n = 250)
70.0
6.4
R


