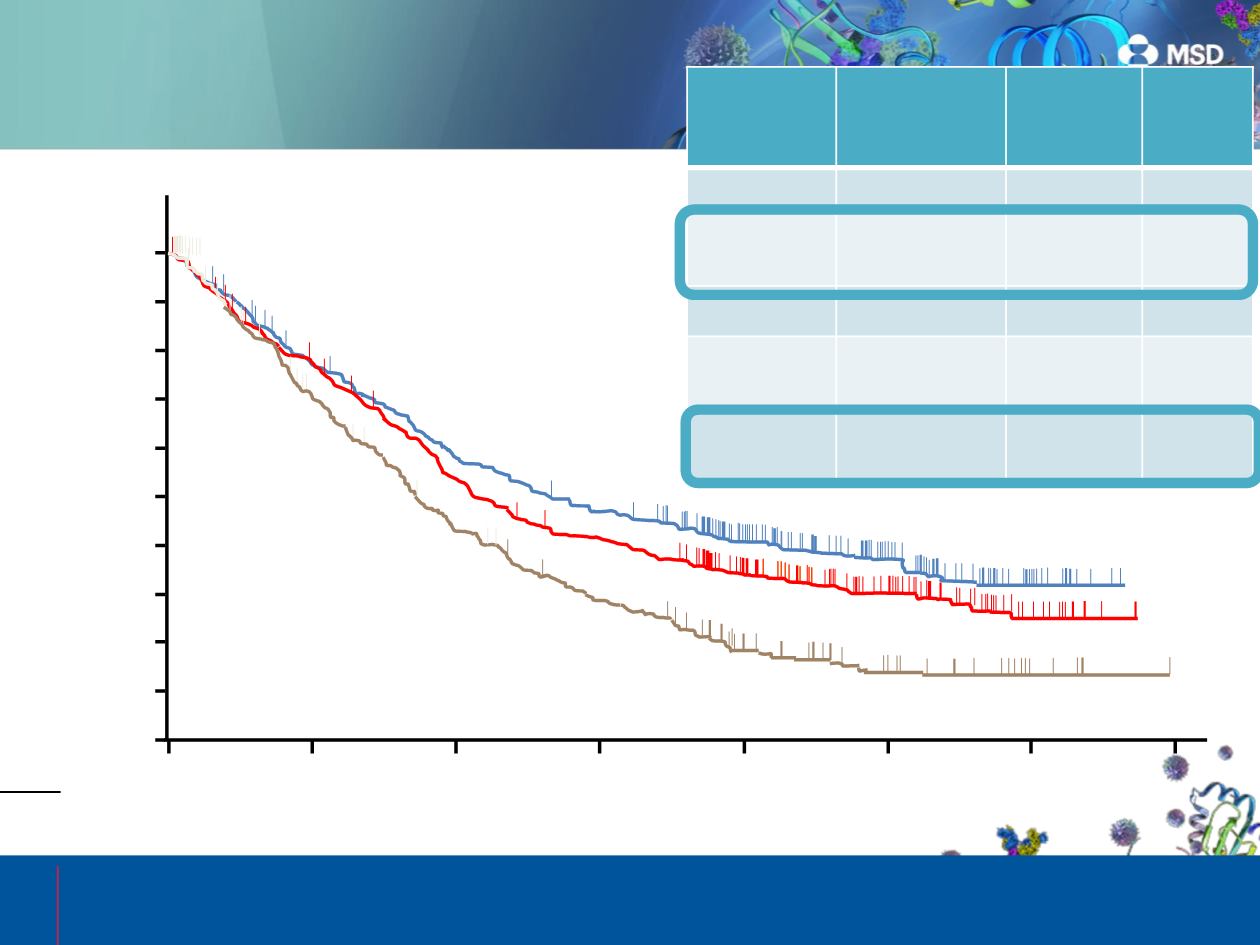
OS in the Total Population
a
a
Comparison of pembrolizumab vs docetaxel. Data are an additional 12 months of follow-
up from the final analysis,
b
Median time from first randomization to current DBL.
Data cutoff date: September 30, 2016.
6769 – RS Herbst, WCLC 2016
Overall Survival, %
Docetaxel 75 mg/m
2
(n = 343)
Pembrolizumab 2 mg/kg Q3W (n = 344)
Pembrolizumab 10 mg/kg Q3W (n = 346)
at risk
etaxel
bro 2 mg/kg Q3W
bro 10 mg/kg Q3W
216
261
259
129
176
195
84
135
259
40
88
100
20
46
57
6
12
15
0
0
1
0
10
20
30
40
50
60
70
80
90
100
0
5
10
15
20
25
30
35
Pembro
2 mg/kg Q3W
n = 344
Pembro
10 mg/kg
Q3W
n = 346
Docetaxel
n = 343
Events, n (%)
233 (68)
214 (62)
257 (75)
OS, median mo
(95% CI)
10.5
(9.6-12.4)
13.4
(11.2-17.0)
8.6
(7.9-9.8)
HR (95% CI)
0.72
(0.60-0.86)
0.60
(0.49-0.72)
—
P value
(vs docetaxel)
0.00017
<0.00001
—
24-mo OS rate,
% (95% CI)
30.1
(25.0-35.4)
37.5
(32.2-42.9)
14.5
(10.5-19.2)
Median follow-up:
b
2.1 years
(range, 1.5-3.0 years)


