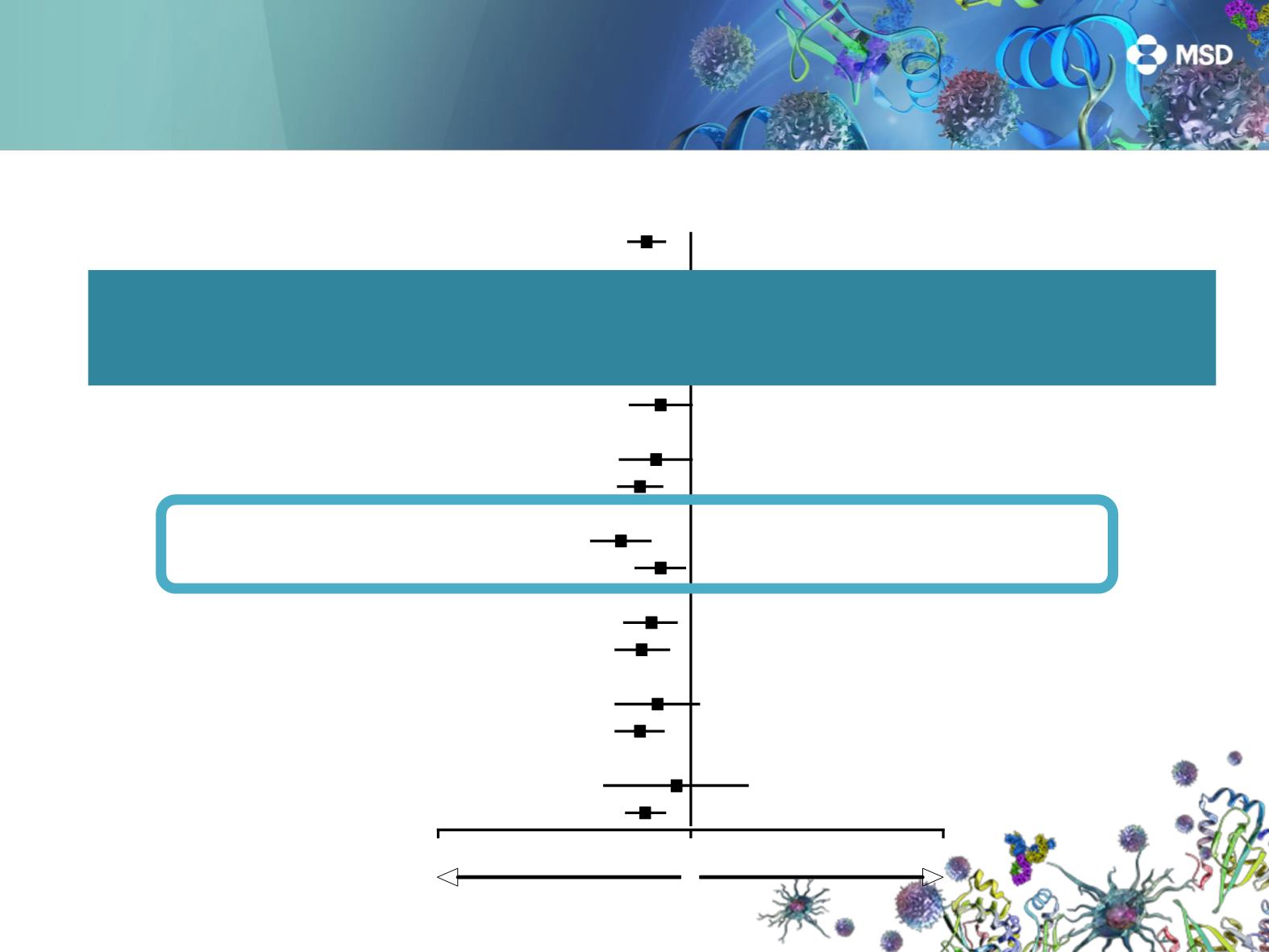
OS in Key Subgroups, PD-L1 TPS ≥1%
a
Analysis cut-off date: September 30, 2015.
a
Data for the pembrolizumab doses were pooled.
0.1
1
10
Overall
Sex
Male
Female
ECOG performance status
0
1
Histology
Squamous
Adenocarcinoma
521/1033
332/634
189/399
149/348
367/678
128/222
333/708
0.67 (0.56-0.80)
0.65 (0.52-0.81)
0.69 (0.51-0.94)
0.73 (0.52-1.02)
0.63 (0.51-0.78)
0.74 (0.50-1.09)
0.63 (0.50-0.79)
Subgroup
No. of Events/
No. of Patients
Hazard Ratio (95% CI)
Favors Pembrolizumab
Favors Docetaxel
PD-L1 tumor proportion score
50%
1%–49%
204/442
317/591
0.53 (0.40-0.70)
0.76 (0.60-0.96)
Age
<65 years
65 years
317/604
204/429
0.63 (0.50-0.79)
0.76 (0.57-1.02)
Tumor sample
Archival
New
266/455
255/578
0.70 (0.54-0.89)
0.64 (0.50-0.83)
EGFR
status
Mutant
Wild type
46/86
447/875
0.88 (0.45-1.70)
0.66 (0.55-0.80)
Confirma que el beneficio de pembrolizumab no se limita a los
pacientes PD-L1 ≥50%, también en pacientes con PD-L1 1-49%.
Herbst RS et al.
Lancet
2016; 387: 1540–1550 (and online appendix).


