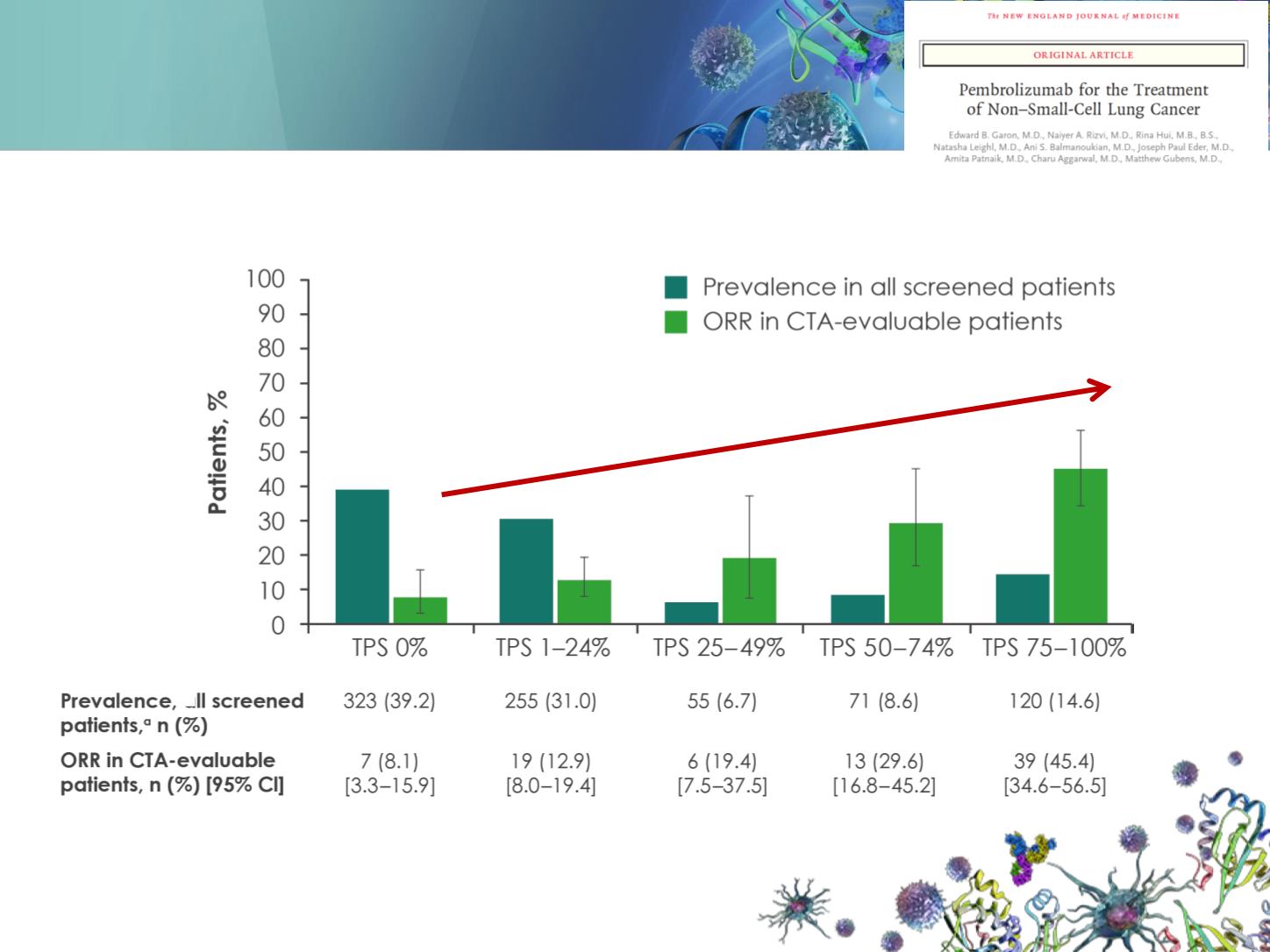
KEYNOTE-001: prevalence of PD-L1 +
and ORR by quartiles of PD-L1 TPS
Prevalence and ORR (RECIST v1.1 by central review) assessed in patients whose samples were evaluable by the CTA, regardless of the interval between cutting
and staining.
CI: confidence interval; CTA: clinical trial assay; ORR: objective response rate;
PD-L1: programmed cell death ligand 1; T
PS: tumour proportion score;
RECIST: Response Evaluation Criteria In Solid Tumors.
Garon EB
et al. N Engl J Med
2015;372:2018–28 (and supplementary appendix).


