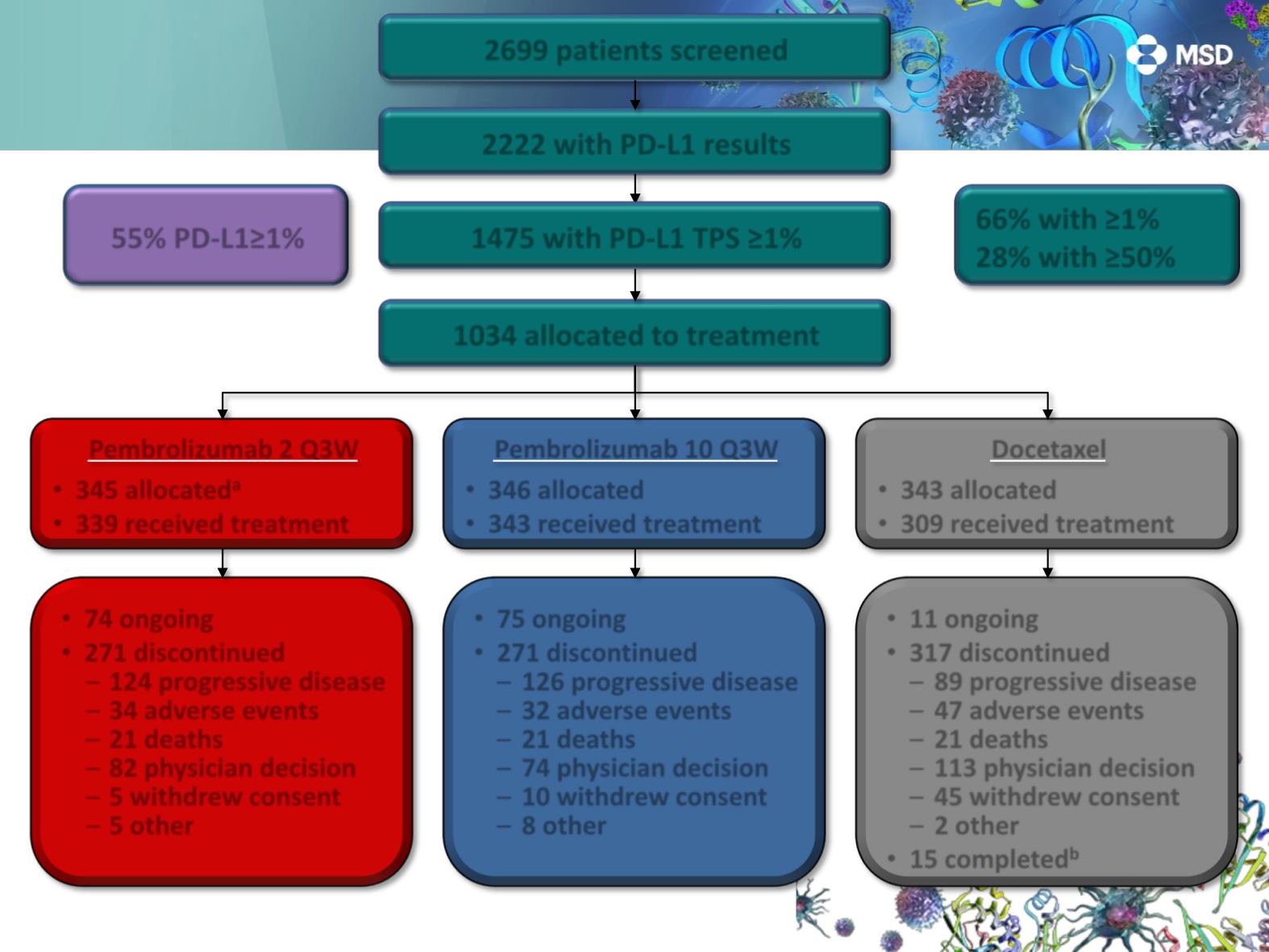
Disposition
1034 allocated to treatment
Pembrolizumab 2 Q3W
•
345 allocated
a
•
339 received treatment
Pembrolizumab 10 Q3W
•
346 allocated
•
343 received treatment
Docetaxel
•
343 allocated
•
309 received treatment
•
74 ongoing
•
271 discontinued
–
124 progressive disease
–
34 adverse events
–
21 deaths
–
82 physician decision
–
5 withdrew consent
–
5 other
•
75 ongoing
•
271 discontinued
–
126 progressive disease
–
32 adverse events
–
21 deaths
–
74 physician decision
–
10 withdrew consent
–
8 other
•
11 ongoing
•
317 discontinued
–
89 progressive disease
–
47 adverse events
–
21 deaths
–
113 physician decision
–
45 withdrew consent
–
2 other
•
15 completed
b
a
1 patient excluded from efficacy analyses because of noncompliance with imaging guidelines for prebaseline scans.
b
Patients who received the maximum number of docetaxel doses permitted per local guidelines.
2222 with PD-L1 results
1475 with PD-L1 TPS ≥1%
66% with ≥1%
28% with ≥50%
2699 patients screened
55% PD-L1≥1%
Herbst RS et al.
Lancet
2016; 387: 1540–1550 (and online appendix).


