KEYNOTE-010
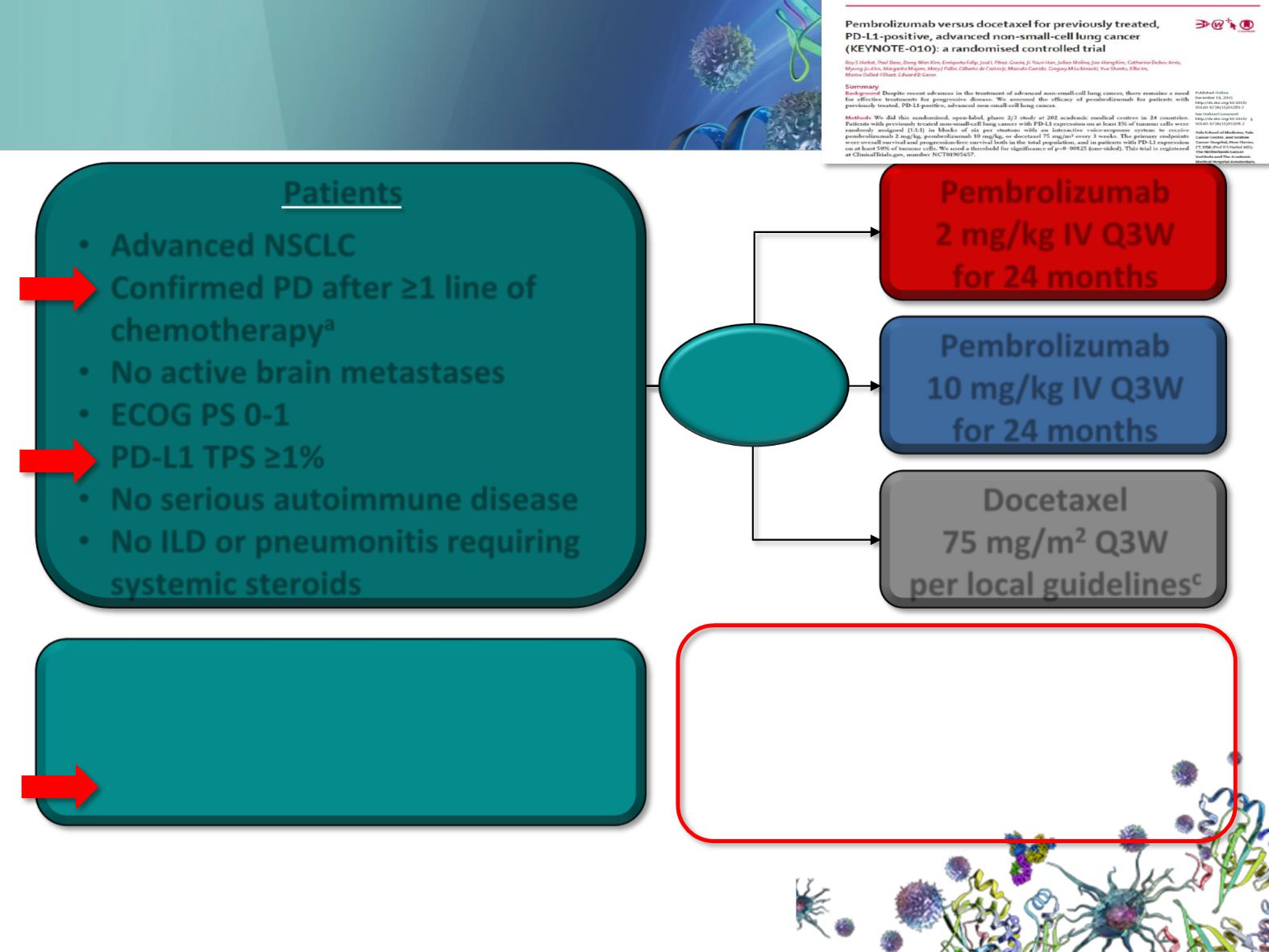
Patients
•
Advanced NSCLC
•
Confirmed PD after ≥1 line of
chemotherapy
a
•
No active brain metastases
•
ECOG PS 0-1
•
PD-L1 TPS ≥1%
•
No serious autoimmune disease
•
No ILD or pneumonitis requiring
systemic steroids
Pembrolizumab
2 mg/kg IV Q3W
for 24 months
Pembrolizumab
10 mg/kg IV Q3W
for 24 months
R
1:1:1
Docetaxel
75 mg/m
2
Q3W
per local guidelines
c
Stratification factors:
•
ECOG PS (0 vs 1)
•
Region (East Asia vs non-East Asia)
•
PD-L1 status
b
(TPS ≥50% vs 1%-49%)
ClinicalTrials.gov, NCT01905657.
a
Prior therapy must have included ≥2 cycles of platinum-doublet chemotherapy. An appropriate tyrosine kinase inhibitor was required for patients whose tumors had an
EGFR
sensitizing mutation or an
ALK
translocation.
b
Added after 441 patients enrolled based on results from KEYNOTE-001 (Garon EB et al.
N Engl J Med
. 2015;372:2018-28).
c
Patients received the maximum number of cycles permitted by the local regulatory authority.
End points in the TPS ≥50% stratum
and TPS ≥1% population
•
Primary: PFS and OS
•
Secondary: ORR, duration of
response, safety
Herbst RS et al.
Lancet
2016; 387: 1540–1550 (and online appendix).


