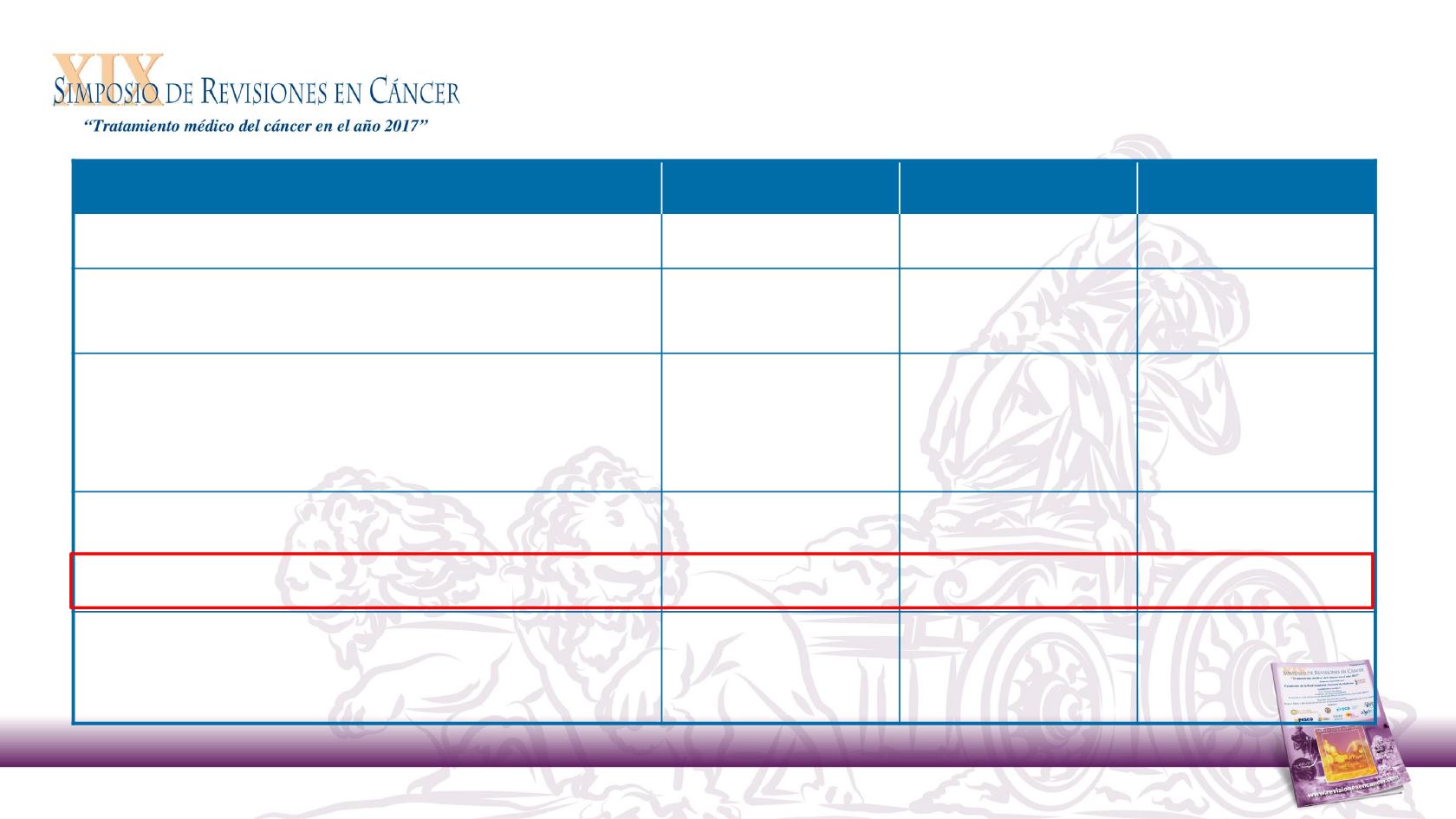
Nivolumab
(n = 240)
Investigator’s Choice
(n = 121)
Total
(N = 361)
Median age, years
59.0
61.0
60.0
<65, n (%)
172 (71.7)
76 (62.8)
248 (68.7)
Smoking/tobacco use, n (%)
Current/former
191 (79.6)
85 (70.2)
276 (76.5)
Never
39 (16.3)
31 (25.6)
70 (19.4)
ECOG performance status, n (%)
0
49 (20.4)
23 (19.0)
72 (19.9)
1
189 (78.8)
94 (77.7)
283 (78.4)
≥2
1 (0.4)
3 (2.5)
4 (1.1)
Not reported
1 (0.4)
1 (0.8)
2 (0.6)
Number of prior lines of systemic cancer therapy, n (%)
1
106 (44.2)
58 (47.9)
164 (45.4)
2
80 (33.3)
45 (37.2)
125 (34.6)
≥3
54 (22.5)
18 (14.9)
72 (19.9)
p16 status
a,b
, n (%)
Positive
63 (26.3)
29 (24.0)
92 (25.5)
Negative
50 (20.8)
36 (29.8)
86 (23.8)
Not tested
127 (52.9)
56 (46.3)
183 (50.7)
Demographics
Nivolumab in R/M SCCHN After Platinum Therapy
20
a
Required from patients with oropharyngeal cancer only.
b
Determined via p16 immunohistochemistry.
ECOG = Eastern Cooperative Oncology Group.


