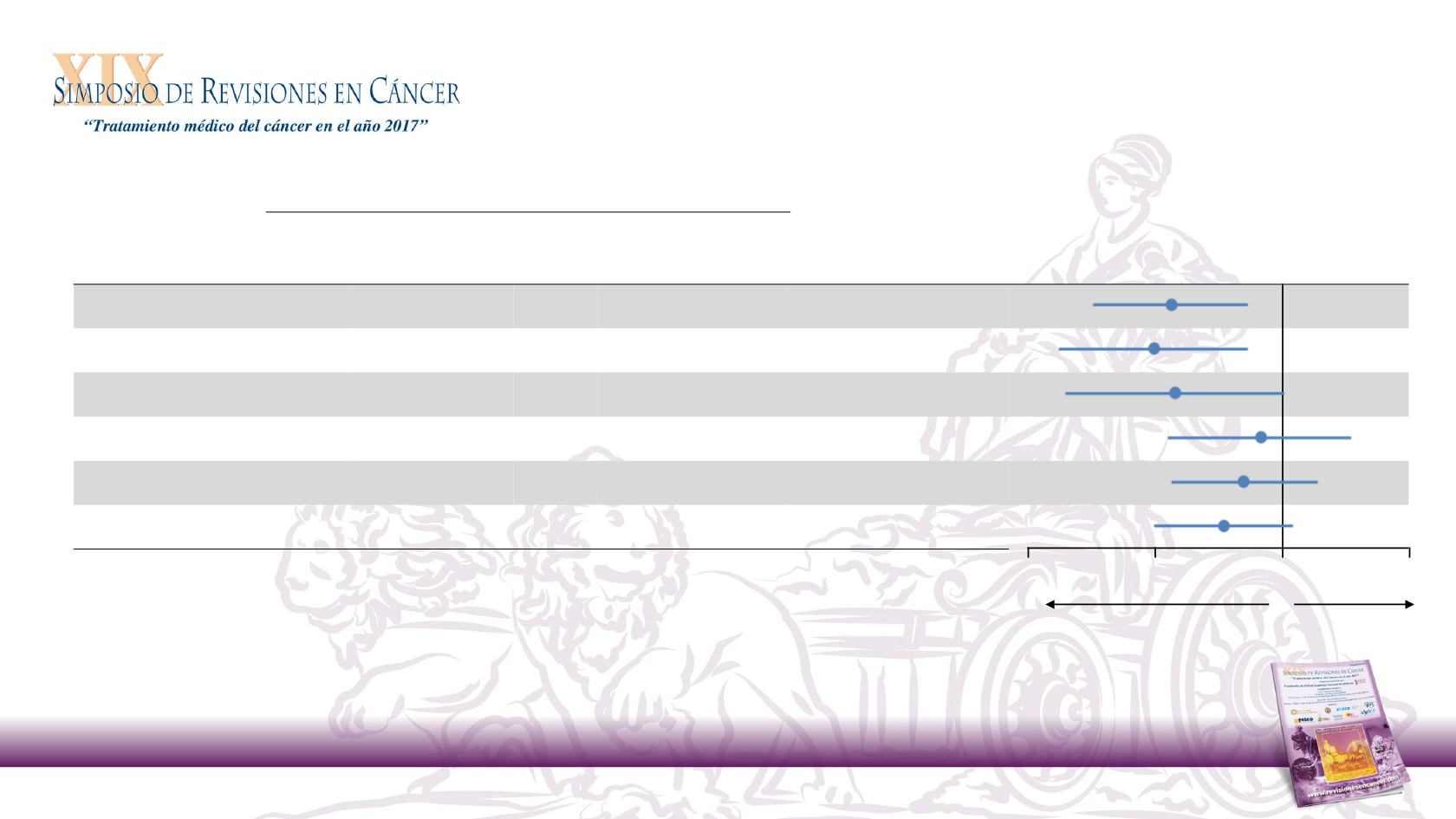
29
PD-L1
Expression
Nivolumab
n = 240
Investigator’s Choice
n = 121
n
Median OS,
mo
n
Median OS,
mo
Unstratified Hazard Ratio (95% CI)
≥ 1%
88
8.7
61
4.6
0.55 (0.36, 0.83)
≥ 5%
54
8.8
43
4.6
0.50 (0.30, 0.83)
≥ 10%
43
8.7
34
5.2
0.56 (0.31, 1.01)
< 1%
73
5.7
38
5.8
0.89 (0.54, 1.45)
< 5%
107
7.0
56
5.1
0.81 (0.55, 1.21)
< 10%
118
7.2
65
4.6
0.73 (0.50, 1.06)
Favors
Nivolumab
Favors
Investigator’s
Choice
•
The magnitude of OS benefit of nivolumab vs
investigator’s choice was greater in patients with
tumor PD-L1 expression
•
Increasing PD-L1 expression did not result in further benefit
0.25
0.5
1
2
Overall Survival by Tumor PD-L1 Expression Level
Nivolumab in R/M SCCHN After Platinum Therapy


