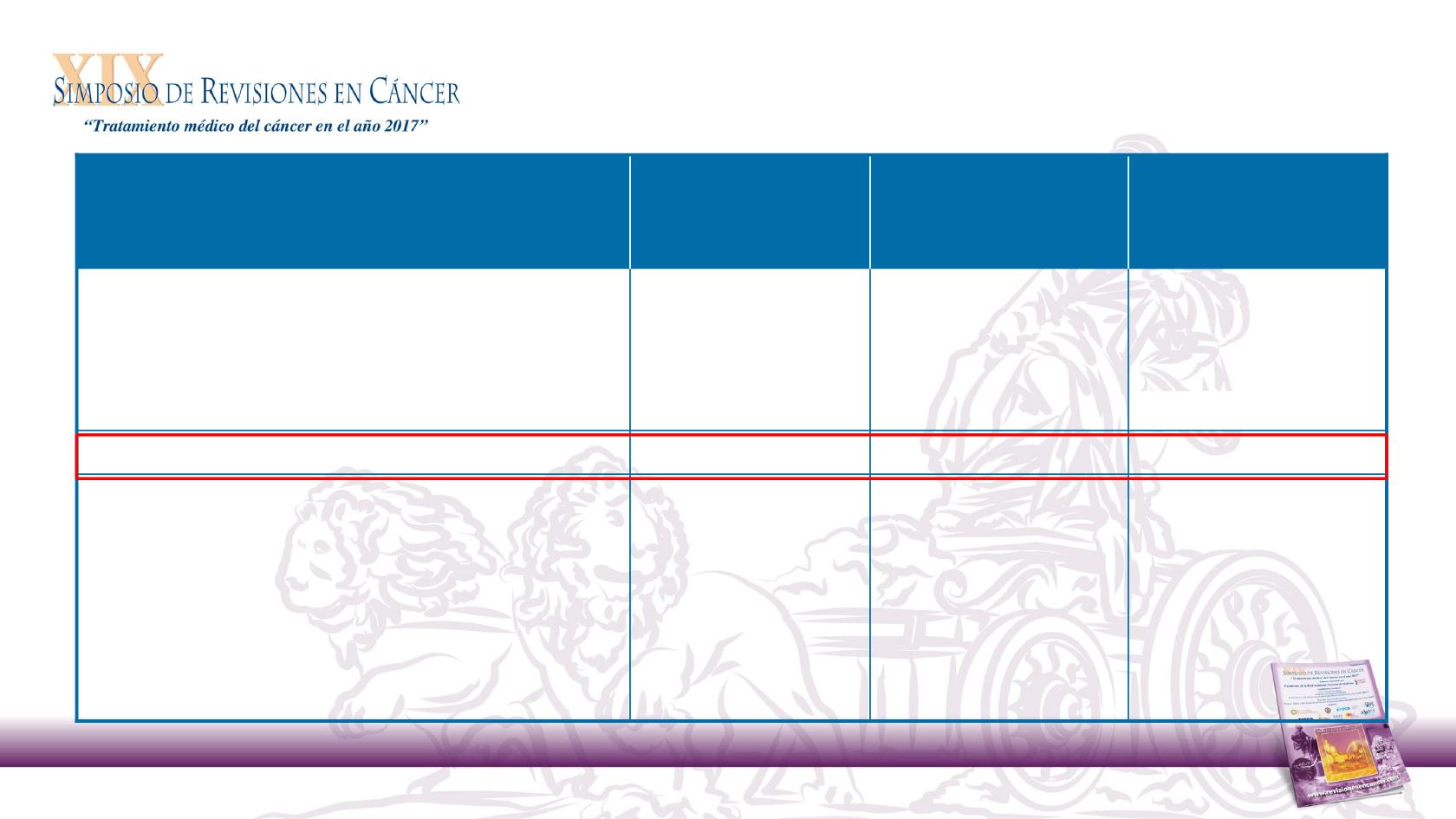
Nivolumab
(n = 240)
Investigator’s
Choice
(n = 121)
Total
(N = 361)
Investigator’s choice therapy, n (%)
Methotrexate
–
52 (43.0)
–
Docetaxel
–
54 (44.6)
–
Cetuximab
–
15 (12.4)
–
Ongoing treatment, n (%)
41 (17.4)
3 (2.7)
44 (12.7)
Not continuing treatment, n (%)
195 (82.6)
108 (97.3)
303 (87.3)
Disease progression
162 (68.6)
83 (74.8)
245 (70.6)
Study drug toxicity
9 (3.8)
11 (9.9)
20 (5.8)
Adverse event not related to study drug
12 (5.1)
3 (2.7)
15 (4.3)
Other
a
9 (3.8)
11(9.9)
20 (5.8)
Not reported
3 (1.3)
0
3 (0.9)
Treatment Administration and Patient Disposition
Nivolumab in R/M SCCHN After Platinum Therapy
a
Other includes patient request to discontinue, withdrawal of consent, non-compliance and maximum clinical benefit.


