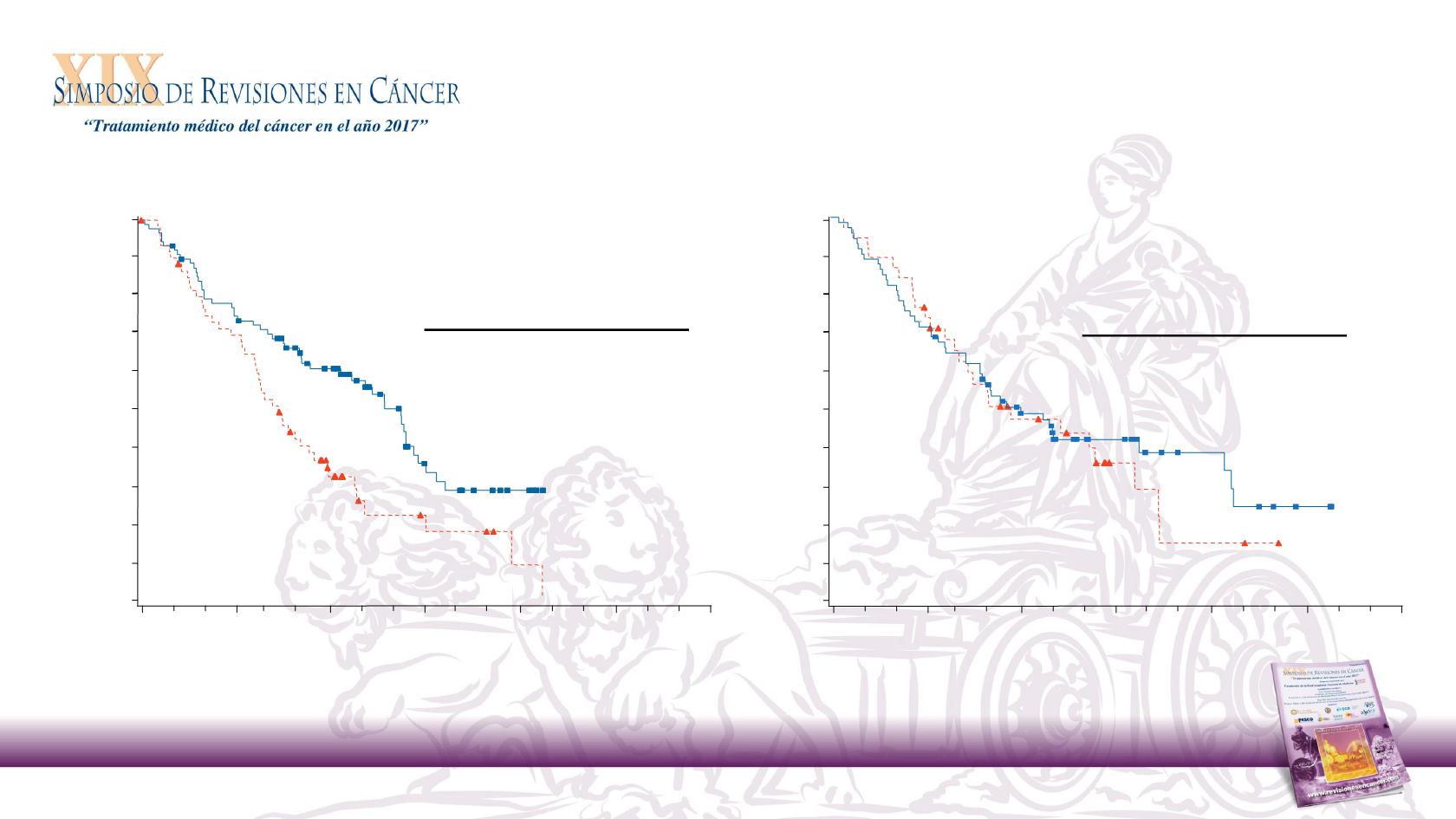
28
Investigator’s Choice (n = 61)
Nivolumab (n = 88)
PD-L1 ≥ 1%
PD-L1 < 1%
HR (95% CI)
0.55 (0.36, 0.83)
Overall Survival (% of patients)
Nivolumab (n = 73)
Investigator’s Choice (n = 38)
HR (95% CI)
0.89 (0.54, 1.45)
Months
0
3
6
9
12
15
18
0
10
20
30
40
50
60
70
80
90
100
Overall Survival (% of patients)
Nivolumab
Investigator’s
Choice
No. at Risk
88
67
44
61
42
20
18
6
6
2
0
0
73
52
33
0
38
29
14
0
17
6
8
2
3
0
Months
0
3
6
9
12
15
18
0
10
20
30
40
50
60
70
80
90
100
Overall Survival by Tumor PD-L1 Expression at 1%
Nivolumab in R/M SCCHN After Platinum Therapy


