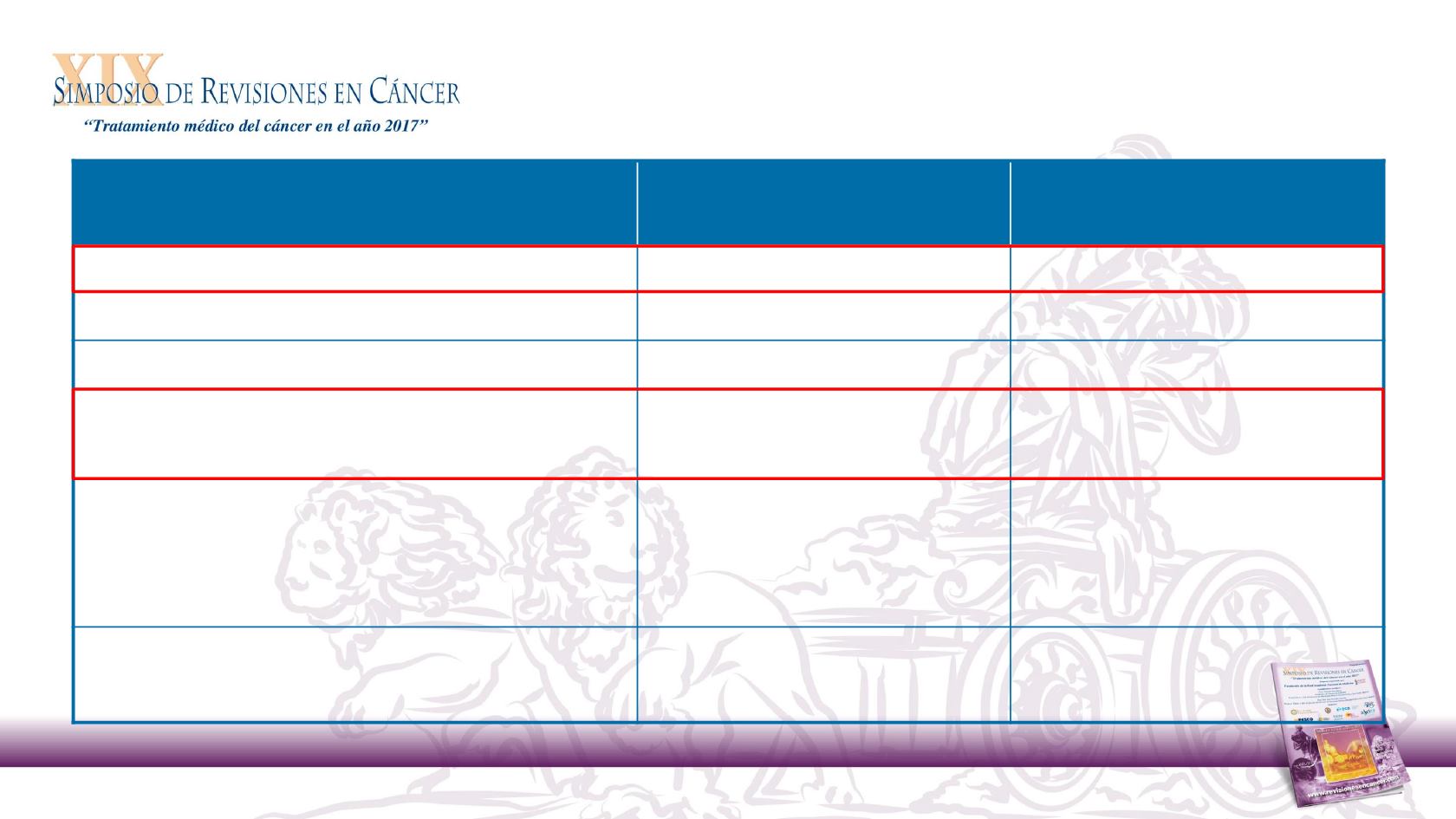
Objective Response Rate
Nivolumab in R/M SCCHN After Platinum Therapy
26
Nivolumab
(n = 240)
Investigator’s Choice
(n = 121)
Objective response rate, n (%)
32 (13.3)
7 (5.8)
95% CI
9.3, 18.3
2.4, 11.6
Best overall response, n (%)
Complete response
6 (2.5)
1 (0.8)
Partial response
26 (10.8)
6 (5.0)
Stable disease
55 (22.9)
43 (35.5)
Progressive disease
100 (41.7)
42 (34.7)
Not determined
53 (22.1)
29 (24.0)
Time to response, mo
Median (range)
2.1 (1.8–7.4)
2.0 (1.9–4.6)


