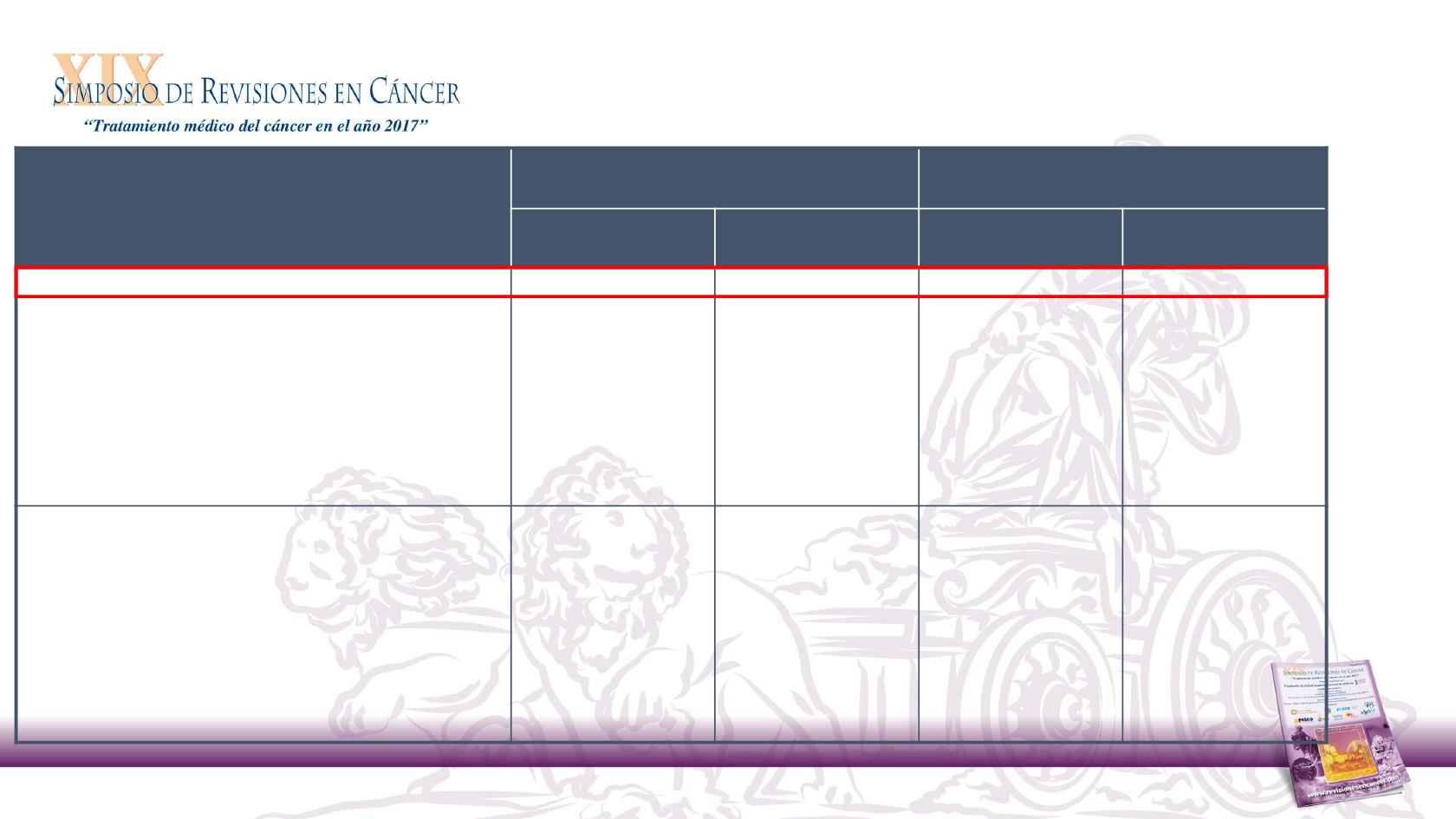
Event
Nivolumab
(n = 236)
Investigator’s Choice
(n = 111)
Any grade
n (%)
Grade 3–4
n (%)
Any grade
n (%)
Grade 3–4
n (%)
Any treatment-related AE in ≥ 10% of patients
a
139 (58.9)
31 (13.1)
86 (77.5)
39 (35.1)
Fatigue
33 (14.0)
5 (2.1)
19 (17.1)
3 (2.7)
Nausea
20 (8.5)
0
23 (20.7)
1 (0.9)
Diarrhea
16 (6.8)
0
15 (13.5)
2 (1.8)
Anemia
12 (5.1)
3 (1.3)
18 (16.2)
5 (4.5)
Asthenia
10 (4.2)
1 (0.4)
16 (14.4)
2 (1.8)
Mucosal inflammation
3 (1.3)
0
14 (12.6)
2 (1.8)
Alopecia
0
0
14 (12.6)
3 (2.7)
Treatment-related select AEs
Skin
37 (15.7)
0
14 (12.6)
2 (1.8)
Endocrine
18 (7.6)
1 (0.4)
1 (0.9)
0
Gastrointestinal
16 (6.8)
0
16 (14.4)
2 (1.8)
Hepatic
5 (2.1)
2 (0.8)
4 (3.6)
1 (0.9)
Pulmonary
5 (2.1)
2 (0.8)
1 (0.9)
0
Hypersensitivity/infusion reaction
3 (1.3)
0
2 (1.8)
1 (0.9)
Renal
1 (0.4)
0
2 (1.8)
1 (0.9)
a
One Grade 5 event (hypercalcemia) in the nivolumab arm and one Grade 5 event (lung infection) in the investigator’s choice arm were reported. A second death occurred in the nivolumab arm
subsequent to pneumonitis
.
Treatment-Related Adverse Events
Nivolumab in R/M SCCHN After Platinum Therapy


