Panitumumab outcome by tumour
location analysis
Background
•
1. Boeckx N, et al. Ann Oncol 2016;27(Suppl 6):abstract 89P (and poster); 2. Douillard JY, et al. N Engl J Med 2013;369:1023‒34;
3. Schwartzberg LS, et al. J Clin Oncol 2014;32:2240‒7; 4. Peeters M, et al. Clin Cancer Res 2015;21:5469‒79.
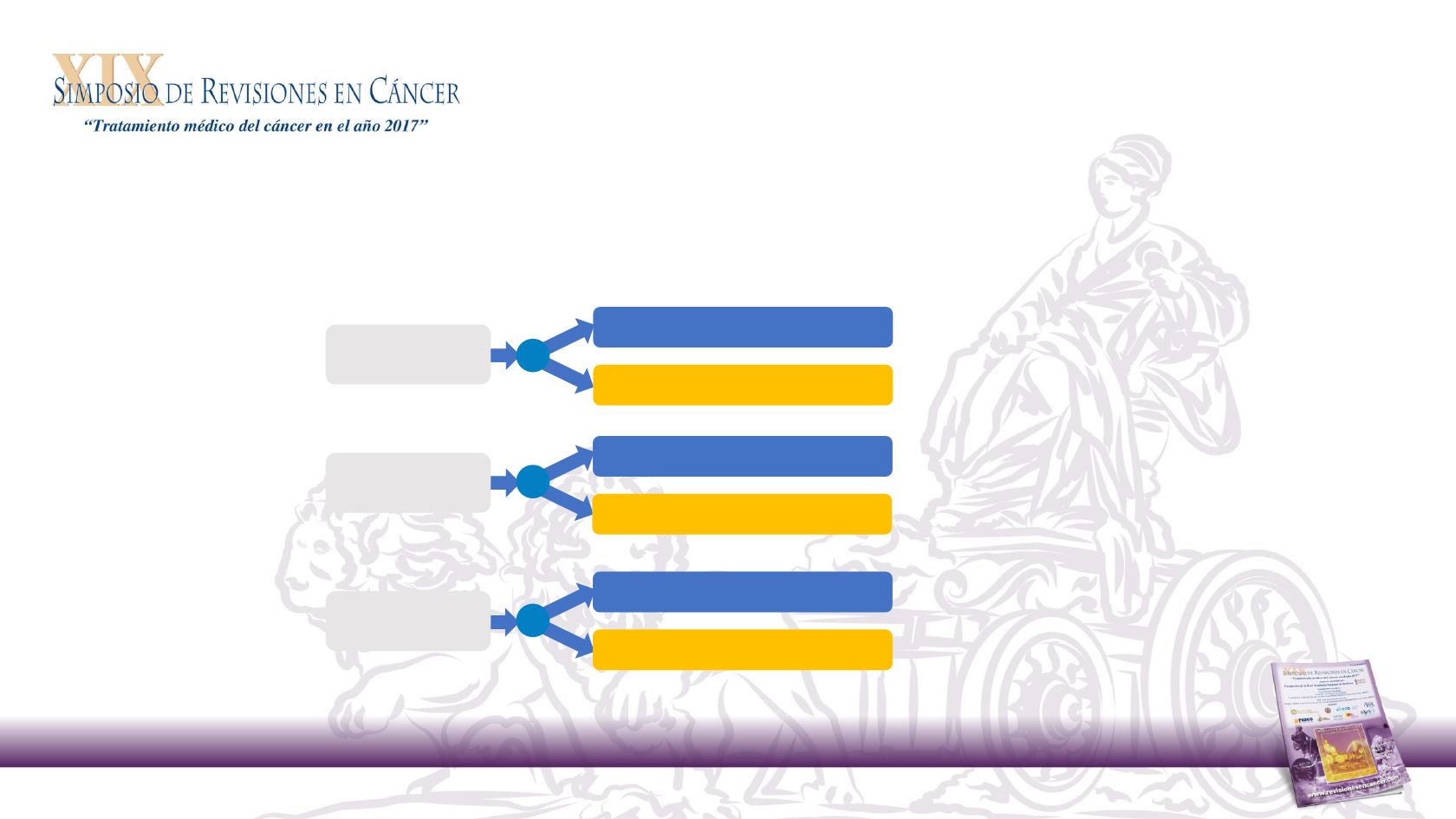
Retrospective, exploratory analysis of outcomes by primary tumour location for
patients with WT
RAS
and WT
RAS
/WT
BRAF
mCRC participating in:
1
Phase 3
1
st
-line mCRC
Panitumumab + FOLFOX4
20050181
4
(NCT00339183)
PEAK
3
(NCT00819780)
PRIME
2
(NCT00364013)
FOLFOX4
Panitumumab + FOLFIRI
FOLFIRI
Panitumumab + mFOLFOX6
Bevacizumab + mFOLFOX6
Phase 2
1
st
-line mCRC
Phase 3
2
nd
-line mCRC
R
R
R


