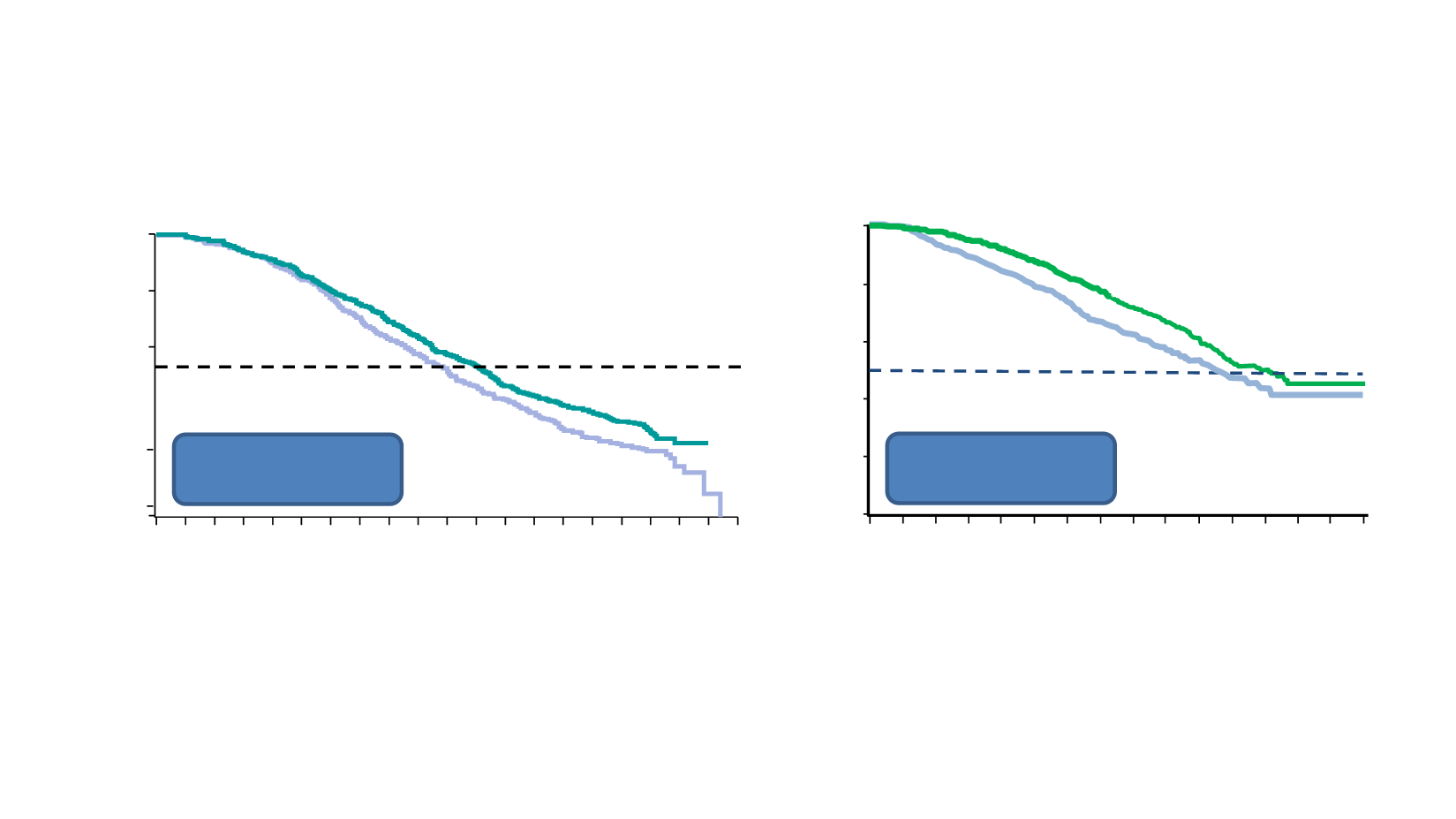
1. Ryan CJ et al. Lancet Oncol 2015;16:152-60; 2. Beer TM et al. Eur Urol 2016:doi: 10.1016/j.eururo.2016.07.032. (epub ahead of print)
AR-targeted agents in chemonaive mCRPC
100
80
60
40
20
0
0
Overall Survival (%)
9
21
30
48
60
39
546
542
525
509
422
401
296
261
59
42
0
0
ABI + P
P
202
148
Time to Death (Months)
24
12
3
36
45
54
538
534
453
438
359
322
189
132
15
10
HR = 0.81 (0.70-0.93)
P
=0.0033
Prednisone (P)
Median 30.3 mo
6
15 18
27
33
42
51
57
0
1
118
84
218
176
504
493
483
466
394
363
330
292
273
227
235
201
ABI + P
Median 34.7 mo
COU-AA-302 (final OS analysis)
1
Phase III randomized controlled trial
Co-primary end-point: OS and rPFS
100
80
60
40
20
0
0
Overall Survival (%)
9
21
30
45
39
872
845
850
782
710
612
289
254
ENZA
Placebo
2
2
Time to death (Months)
24
12
3
36
863
835
758
657
597
504
HR = 0.77 (0.67-0.88)
P
=0.0002
Placebo
Median 31.3 mo
6
15 18
27
33
42
21
16
824
745
798
702
665
551
441
365
174
153
86
72
ENZA
Median 35.3 mo
0
0
Phase III randomized controlled trial.
Co-primary end-point: OS and rPFS
PREVAIL (final OS analysis)
2
SAEU.CAB.16.07.0040j


