0
10
20
30
40
50
60
70
80
90
100
0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48
Number at risk
CABA 20 +P
598
469 393 324
210
109
55
30
9
0
CABA 25 +P
602
494 416 338
219
120
58
25
11
0
Probability of OS (%)
Time (months)
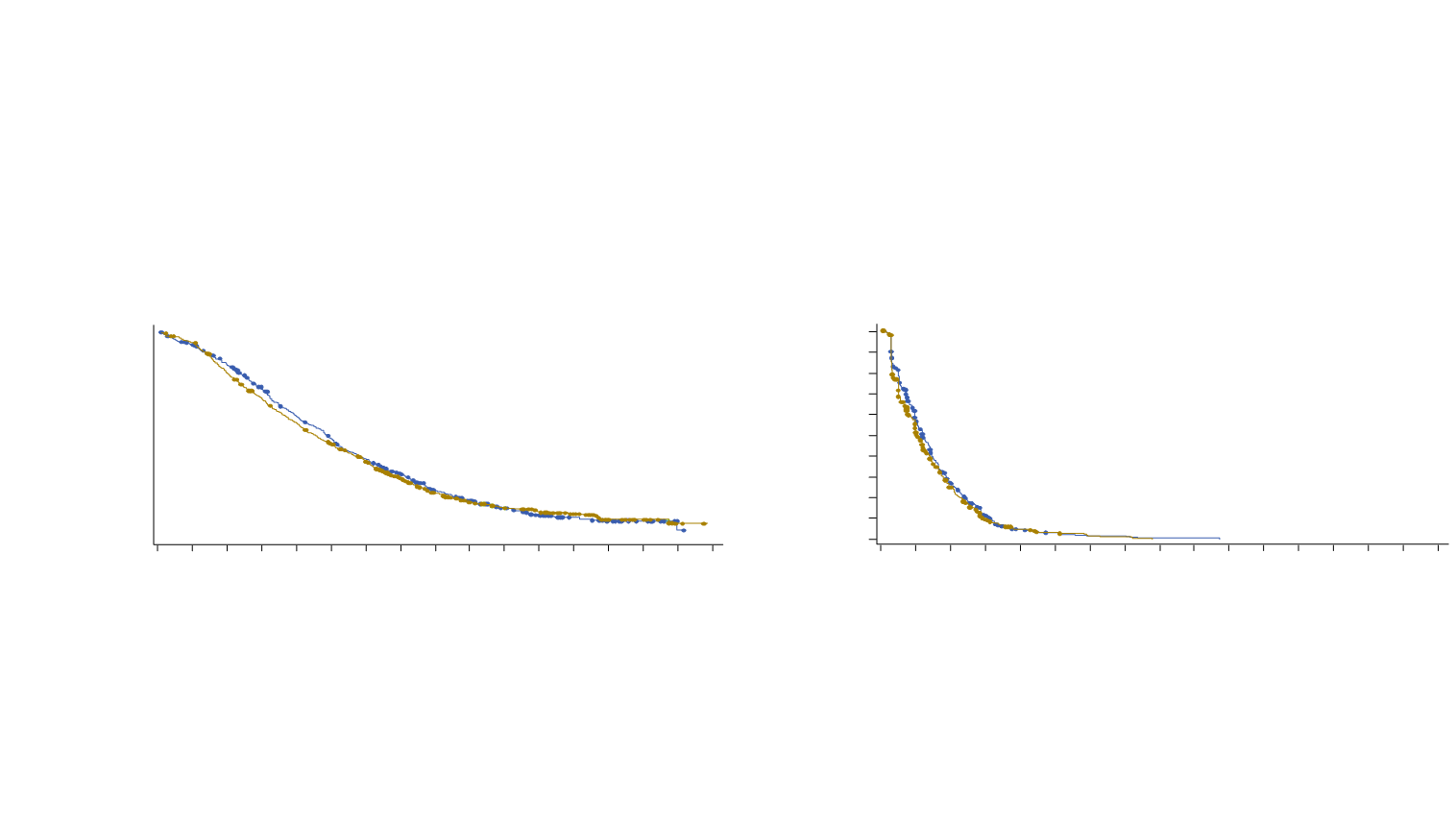
OS (primary endpoint)
PFS (composite)*
Median OS
CABA 20 + P
13.4 mths
CABA 25 + P
14.5 mths
Median PFS
CABA 20 + P
2.9 mths
CABA 25 + P
3.5 mths
HR (
20
vs
25
) = 1.099 (0.974-1.24)
HR (
20
vs
25
) = 1.024
one-sided 98.9% upper-bound CI: 1.184
within the non-inferiority margin (1.214)
0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48
Number at risk
CABA 20 +P
598
123 39 16
4
0
0
0
0
CABA 25 +P
602
136 49 14
4
1
0
0
0
Probability of PFS (%)
Time (months)
0
10
20
30
40
50
60
70
80
90
100
De Bono J et al. J Clin Oncol 2016;34(suppl):abstract 5008 - ClinicalTrials.gov NCT01308580
PROSELICA - Randomized, non-inferiority
phase III trial of CABA (2 doses) after DOC
*PFS: progression-free survival defined as tumor progression or PSA progression or pain progression or death
Randomized multicenter open-label phase III trial comparing 2 doses of CABA (20 vs 25 mg/m
2
q3w) in 1,200
mCRPC pts previously treated with DOC. Primary end-point: OS.
Non-inferiority design (hypothesis that CABA
20 maintains at least 50% of the OS benefit of CABA 25 vs mitoxantrone in TROPIC trial)
SAEU.CAB.16.07.0040j


