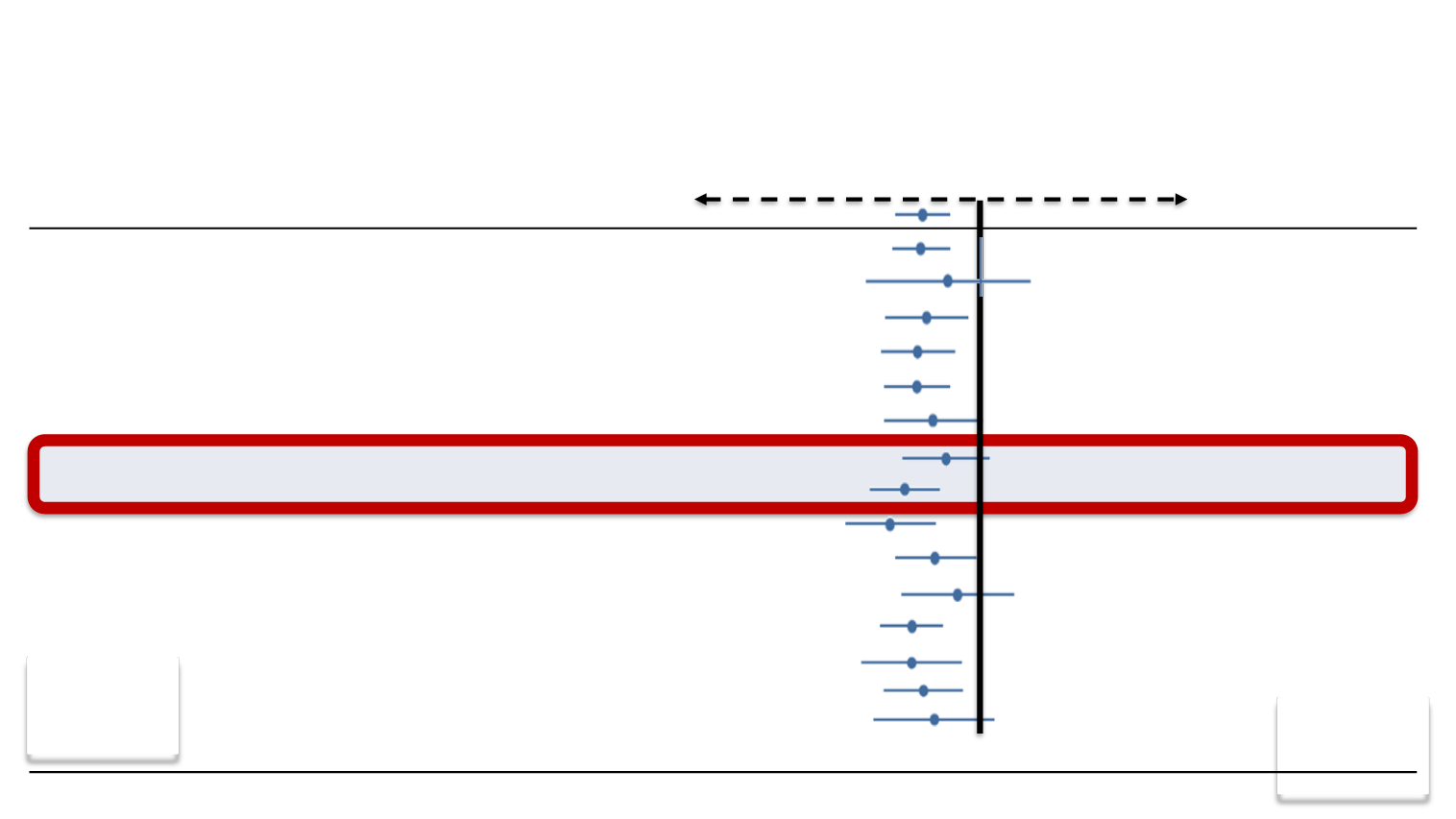
37
Subgroup
N
Favors Cabazitaxel Favors Placebo
HR (95% CI)
All randomized patients
755
0.70 (0.59-0.83)
ECOG status: no
ECOG status: yes
694
61
0.68 (0.57-0.82)
0.81 (0.48-1.38)
Mesurable disease: no
Mesurable disease: yes
350
405
0.72 (0.55-0.93)
0.68 (0.54-0.85)
Number of previous chemotherapies: 1
Number of previous chemotherapies: ≥2
528
227
0.67 (0.55-0.83)
0.75 (0.55-1.02)
Age <65 years
Age ≥65 years
295
460
0.81 (0.61-1.08)
0.62 (0.50-0.78)
Pain at baseline: no
Pain at baseline: yes
314
310
0.57 (0.43-0.77)
0.76 (0.59-0.98)
Rising PSA at baseline: no
Rising PSA at baseline: yes
159
583
0.88 (0.61-1.26)
0.65 (0.53-0.80)
Progression during docetaxel treatment
Progression <3 months after docetaxel
Progression ≥3 months after docetaxel
219
339
192
0.65 (0.47-0.90)
0.70 (0.55-0.91)
0.75 (0.51-1.11)
de Bono JS et al. Lancet 2010;376:1147-54
TROPIC: OS is not influenced by age
1
2
0.5
0.25
SAEU.CAB.16.07.0040j


