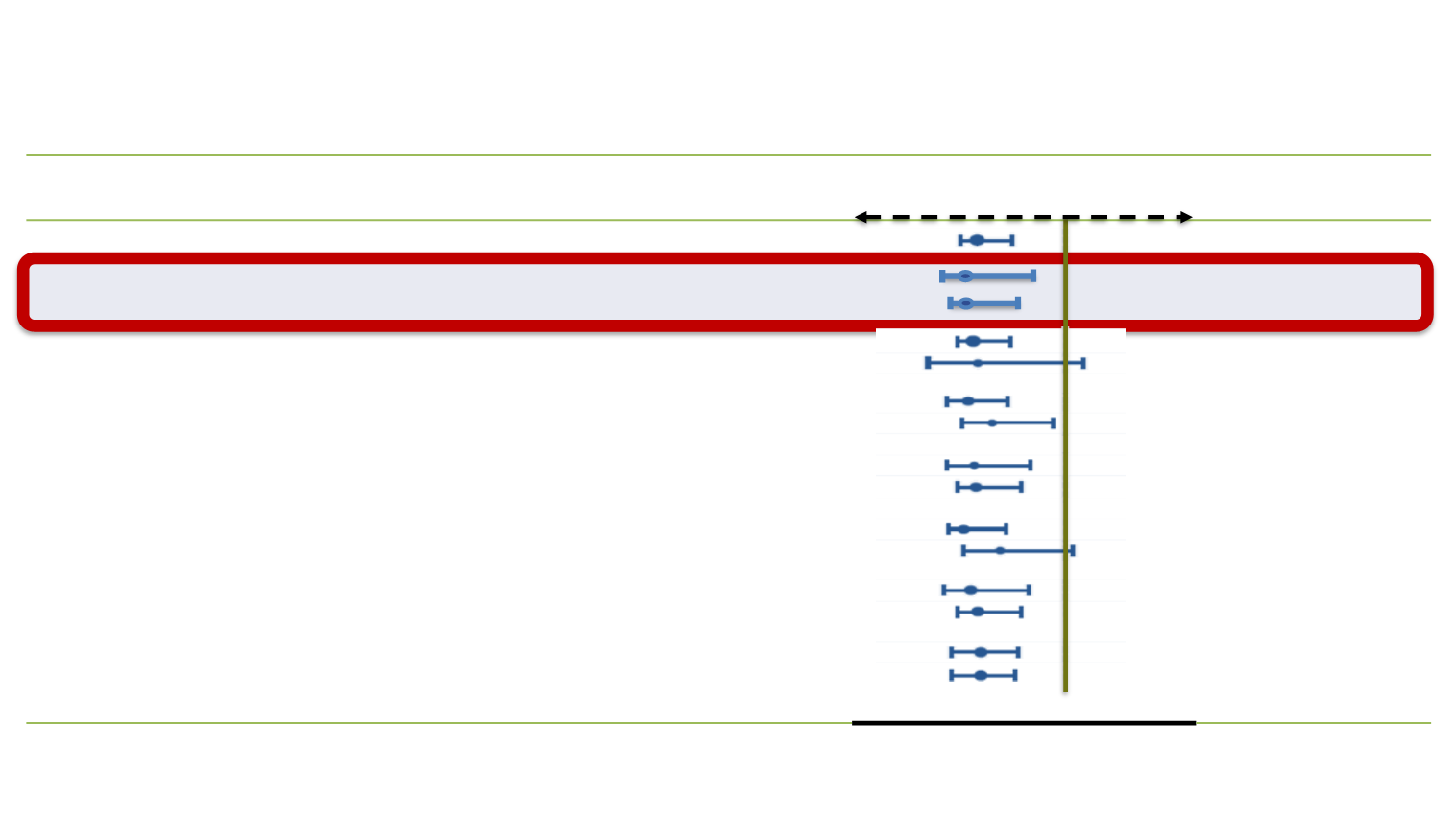
Subgroup
ENZA
Median (mo)
Placebo
Median (mo)
Favors
ENZA
Favors
Placebo
HR (95% CI)
All subjects
18.4
13.6
0.63 (0.53-0.75)
Age <65 years
Age ≥65 years
-
18.4
12.6
13.9
0.63 (0.46-0.87)
0.63 (0.51-0.78)
Baseline ECOG PS score: 0-1
Baseline ECOG PS score: 2
-
10.5
14.2
7.2
0.62 (0.52-0.75)
0.65 (0.51-0.78)
Baseline BPI-SF score <4
Baseline BPI-SF score ≥4
-
12.4
16.2
9.1
0.59 (0.47-0.74)
0.71 (0.54-0.94)
Geographic region: North America
Geographic region: Other
17.4
-
12.3
14.4
0.63 (0.47-0.83)
0.64 (0.51-0.80)
Number of prior CT regimens: 1
Number of prior CT regimens: ≥2
-
15.9
14.2
12.3
0.59 (0.48-0.73)
0.74 (0.54-1.03)
Type of progression: PSA only
Type of progression: Radiographic
-
17.3
19.5
13.0
0.62 (0.46-0.83)
0.64 (0.52-0.80)
Baseline value >median value: PSA
Baseline value >median value: LDH
15.3
12.4
10.3
8.5
0.62 (0.50-0.78)
0.61 (0.50-0.76)
ENZA: enzalutamide; CT: chemotherapy ECOG: Eastern cooperative oncology group; BPI-SF: brief pain inventory – short form; LDH: lactate hydrogenase
Scher HI et al. N Engl J Med 2012;367:1187-97
AFFIRM: OS not influenced by age
1
1.5
0.5
0.0
SAEU.CAB.16.07.0040j


