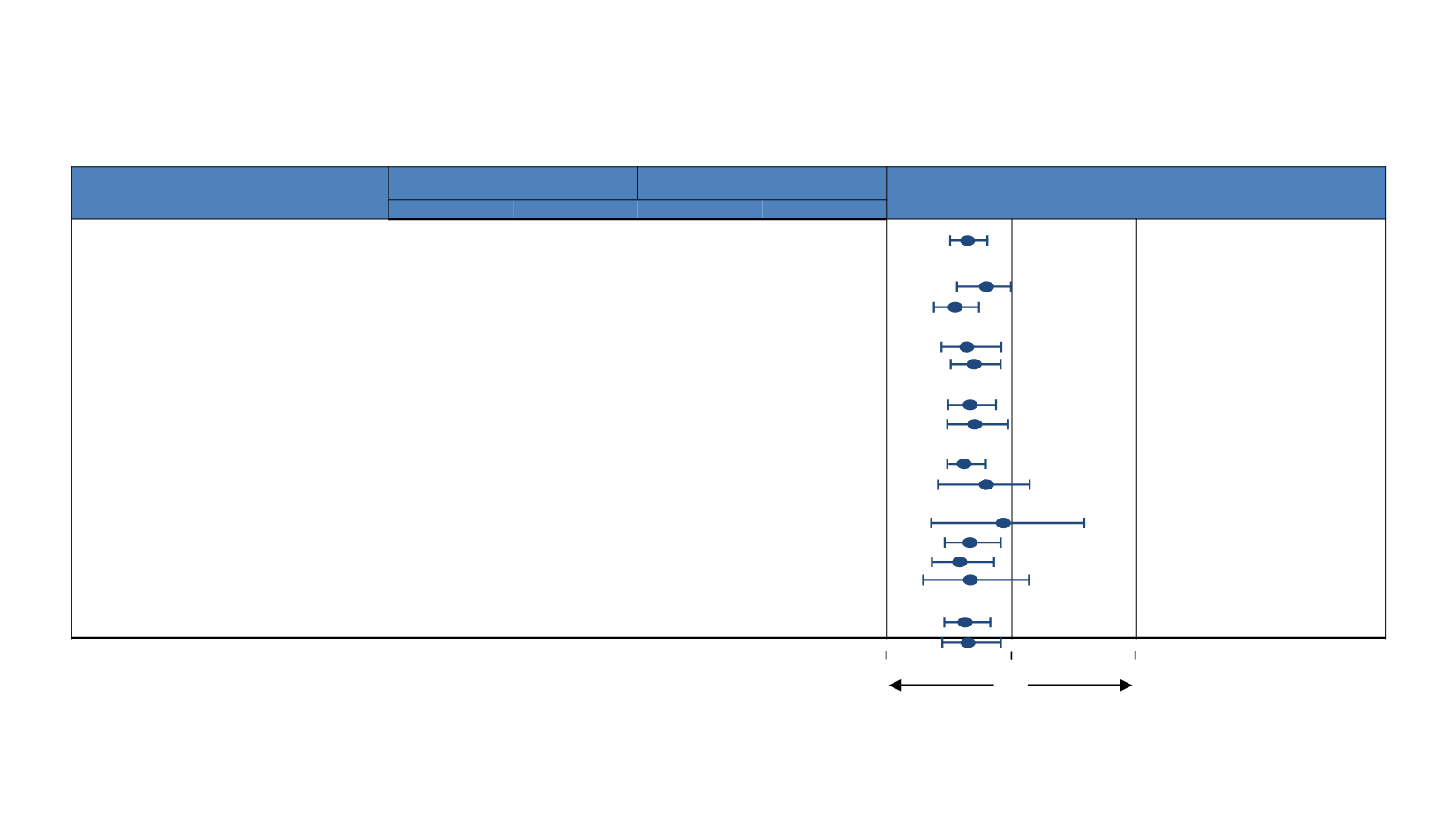
Radium 223:
Subgroups
Subgroup
Number of Patients
Median Overall Survival (months)
Hazard Ratio (95% CI)
Radium 223
Placebo
Radium 223
Placebo
All patients
614
307
14.9
11.3
0.70
0.58-0.83
Total ALP
<220 U/L
348
169
17.0
15.8
0.82
0.64-1.07
≥220 U/L
266
138
11.4
8.1
0.62
0.49-0.79
Current use of bisphosphonates
Yes
250
124
15.3
11.5
0.70
0.52-0.93
No
364
183
14.5
11.0
0.74
0.59-0.92
Prior use of docetaxel
Yes
352
174
14.4
11.3
0.71
0.56-0.89
No
262
133
16.1
11.5
0.74
0.56-0.99
Baseline ECOG performance status
0 or 1
536
265
15.4
11.9
0.68
0.56-0.82
≥2
77
41
10.0
8.4
0.82
0.50-1.35
Extent of disease
<6 Metastases
100
38
27.0
NE
0.95
0.46-1.95
6-20 Metastases
262
147
13.7
11.6
0.71
0.54-0.92
>20 Metastases
195
91
12.5
9.1
0.64
0.47-0.88
Superscan
54
30
11.3
7.1
0.71
0.40-1.27
Opioid use
Yes
a
345
168
13.9
10.4
0.68
0.54-0.86
No
b
269
139
16.4
12.8
0.70
0.52-0.93
0.5
1
Favors
Radium 223
Favors
Placebo
2


